Carbocations
For reactivities of structurally related compounds go to: Electrophiles » C-Electrophiles
Found 134 Molecules.
| Molecule | Solvent | Reactivity Parameters | Classification | Reference (sort by title or year) |
|---|---|---|---|---|
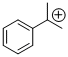 cumyl cation | E Parameter: 5.74 | Macromolecules 2010, 43, 1719-1723 DOI: 10.1021/ma9024569 | ||
 (4-CF3)2-tritylium ion | E Parameter: 2.28 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (3-Cl)3-tritylium ion | E Parameter: 1.99 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-CF3)-tritylium ion | E Parameter: 1.33 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (3-Cl)-tritylium ion | E Parameter: 1.06 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (3-CF3)-tritylium ion | E Parameter: 1.18 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-Me)-tritylium ion | E Parameter: -0.13 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-Me)2-tritylium ion | E Parameter: -0.70 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-Me)3-tritylium ion | E Parameter: -1.21 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-MeO,3-OMe)-tritylium ion | E Parameter: -1.62 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-OMe,3-Me)-tritylium ion | E Parameter: -1.84 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-MeO)-tritylium ion | E Parameter: -1.59 | Eur. J. Org. Chem. 2011, , 6470-6475 DOI: 10.1002/ejoc.201100910 | ||
 (4-MeO,4-Me)-tritylium ion | E Parameter: -2.13 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-MeO)2-tritylium ion | E Parameter: -3.04 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
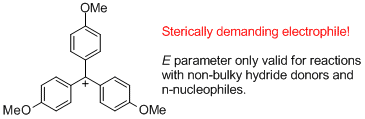 (4-MeO)3-tritylium ion | E Parameter: -4.35 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-NMe2)-tritylium ion | E Parameter: -7.93 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-NMe2)2-tritylium ion (= Malachite green) | E Parameter: -10.29 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
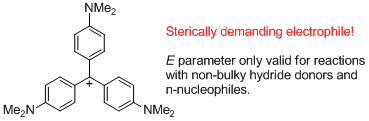 (4-NMe2)3-tritylium ion (= Crystal violet) | E Parameter: -11.26 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | ||
 (4-F)-tritylium ion | E Parameter: 0.35 | Eur. J. Org. Chem. 2011, , 6470-6475 DOI: 10.1002/ejoc.201100910 | ||
 (4-F)2-tritylium ion | E Parameter: 0.17 | Eur. J. Org. Chem. 2011, , 6470-6475 DOI: 10.1002/ejoc.201100910 | ||
 (4-F)3-tritylium ion | E Parameter: 0.05 | Eur. J. Org. Chem. 2011, , 6470-6475 DOI: 10.1002/ejoc.201100910 | ||
 (3-F)-tritylium ion | E Parameter: 1.01 | Eur. J. Org. Chem. 2011, , 6470-6475 DOI: 10.1002/ejoc.201100910 | ||
 (3-F)2-tritylium ion | E Parameter: 1.54 | Eur. J. Org. Chem. 2011, , 6470-6475 DOI: 10.1002/ejoc.201100910 | ||
 (3-F)3-tritylium ion | E Parameter: 2.07 | Eur. J. Org. Chem. 2011, , 6470-6475 DOI: 10.1002/ejoc.201100910 | ||
 (3-F)4-tritylium ion | E Parameter: 2.54 | Eur. J. Org. Chem. 2011, , 6470-6475 DOI: 10.1002/ejoc.201100910 | ||
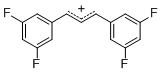 diarylallylium ion (3,3-F2)2 | E Parameter: 6.11 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
 diarylallylium ion (3-F)2 | E Parameter: 4.15 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
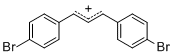 diarylallylium ion (4-Br)2 | E Parameter: 2.85 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
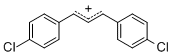 diarylallylium ion (4-Cl)2 | E Parameter: 2.69 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
 diphenylallylium ion | E Parameter: 2.70 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
 di-p-tolyl-allylium ion | E Parameter: 1.23 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
 di-p-anisyl-allylium ion | E Parameter: -1.45 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
 diarylallylium ion (4-NMe2)2 | E Parameter: -7.50 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
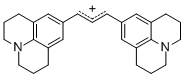 bis(julidin-9-yl)allylium ion | E Parameter: -9.78 | J. Org. Chem. 2011, 76, 9391-9408 DOI: 10.1021/jo201668w | ||
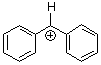 benzhydrylium ion Ph2CH+ | 0 | E Parameter: 5.47 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
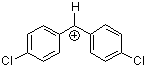 (pcp)2CH+ | 0 | E Parameter: 5.48 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
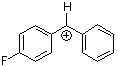 pfp(Ph)CH+ | 0 | E Parameter: 5.20 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
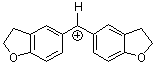 (fur)2CH+ | 0 | E Parameter: -1.36 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
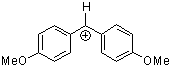 (ani)2CH+ | 0 | E Parameter: 0.00 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
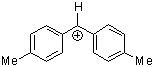 (tol)2CH+ | 0 | E Parameter: 3.63 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
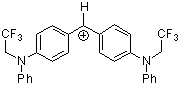 (pfa)2CH+ | 0 | E Parameter: -3.14 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
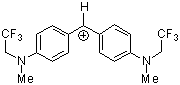 (mfa)2CH+ | 0 | E Parameter: -3.85 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
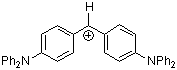 (dpa)2CH+ | 0 | E Parameter: -4.72 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
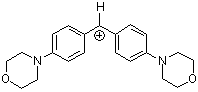 (mor)2CH+ | 0 | E Parameter: -5.53 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
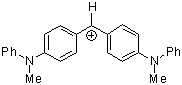 (mpa)2CH+ | 0 | E Parameter: -5.89 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
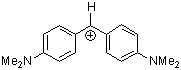 (dma)2CH+ | 0 | E Parameter: -7.02 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
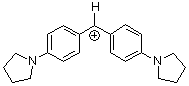 (pyr)2CH+ | 0 | E Parameter: -7.69 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
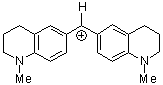 (thq)2CH+ | 0 | E Parameter: -8.22 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
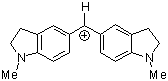 (ind)2CH+ | 0 | E Parameter: -8.76 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
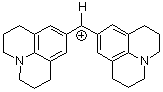 (jul)2CH+ | 0 | E Parameter: -9.45 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
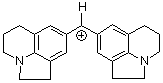 (lil)2CH+ | 0 | E Parameter: -10.04 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
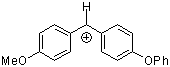 ani(pop)CH+ | 0 | E Parameter: 0.61 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
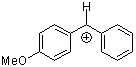 ani(Ph)CH+ | 0 | E Parameter: 2.11 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
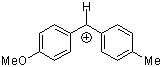 ani(tol)CH+ | 0 | E Parameter: 1.48 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
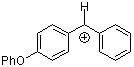 pop(Ph)CH+ | 0 | E Parameter: 2.90 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
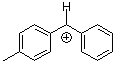 tol(Ph)CH+ | 0 | E Parameter: 4.43 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
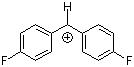 (pfp)2CH+ | 0 | E Parameter: 5.01 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
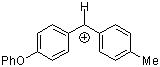 pop(tol)CH+ | 0 | E Parameter: 2.16 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
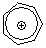 tropylium ion | 0 | E Parameter: -3.72 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
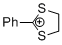 2-phenyl-1,3-dithiolan-2-ylium ion | 0 | E Parameter: -5.91 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
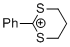 2-phenyl-1,3-dithianylium ion | 0 | E Parameter: -6.43 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
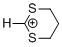 1,3-dithianylium ion | 0 | E Parameter: -2.14 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
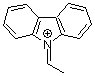 N-ethylidenecarbazolium ion | 0 | E Parameter: 2.41 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
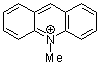 N-methylacridinium ion | 0 | E Parameter: -7.15 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
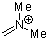 dimethylmethyleneammonium ion | 0 | E Parameter: -6.69 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
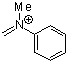 methyl(phenyl)methyleneammonium ion | 0 | E Parameter: -5.17 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
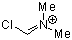 Vilsmeier ion | 0 | E Parameter: -5.77 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
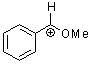 methoxy-phenylmethylium ion | 0 | E Parameter: 2.97 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
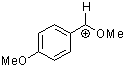 methoxy-(4-methoxyphenyl)methylium ion | 0 | E Parameter: 0.14 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
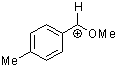 methoxy-(4-methylphenyl)methylium ion | 0 | E Parameter: 1.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
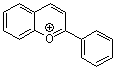 flavylium ion | 0 | E Parameter: -3.45 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
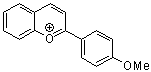 2-(4-methoxyphenyl)chromenylium ion | 0 | E Parameter: -4.95 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
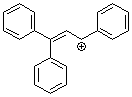 1,1,3-triphenylallylium ion | 0 | E Parameter: 0.98 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
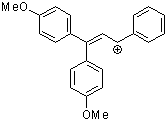 1,1-dianisyl-3-phenylallylium ion | 0 | E Parameter: -2.67 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
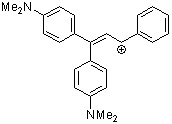 1,1-bis(4-dimethylaminophenyl)-3-phenylallylium ion | 0 | E Parameter: -8.97 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
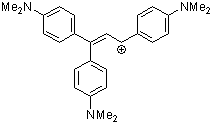 1,1,3-tris(4-(dimethylamino)phenyl)allylium ion | 0 | E Parameter: -9.84 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
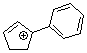 1-phenylcyclopent-2-enylium ion | 0 | E Parameter: 2.89 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
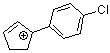 1-(4-chlorophenyl)cyclopent-2-enylium ion | 0 | E Parameter: 3.20 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 tritylium ion (Ph3C+) | 0 | E Parameter: 0.51 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
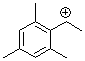 (2,4,6-trimethylphenyl)ethylium ion | 0 | E Parameter: 6.04 | Macromolecules 2005, 38, 33-40 DOI: 10.1021/ma048389o | |
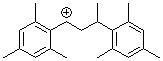 1,3-bis(2,4,6-trimethylphenyl)but-1-ylium ion | 0 | E Parameter: 6.16 | Macromolecules 2005, 38, 33-40 DOI: 10.1021/ma048389o | |
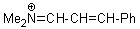 Me2N+=CH-CH-Ph | 0 | E Parameter: -9.20 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 DOI: 10.1002/anie.200802889 | |
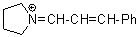 pyrN+=CH-CH-Ph | 0 | E Parameter: -9.80 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 DOI: 10.1002/anie.200802889 | |
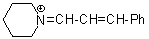 pipN+=CH-CH-Ph | 0 | E Parameter: -10.30 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 DOI: 10.1002/anie.200802889 | |
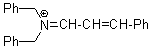 (PhCH2)2N+=CH-CH-Ph | 0 | E Parameter: -8.50 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 DOI: 10.1002/anie.200802889 | |
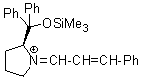 Jorgensen/Hayashi-iminium ion | 0 | E Parameter: -8.20 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 DOI: 10.1002/anie.200802889 | |
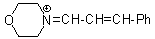 mor iminium | 0 | E Parameter: -8.60 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 DOI: 10.1002/anie.200802889 | |
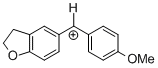 (fur)(ani)CH+ | 0 | E Parameter: -0.81 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
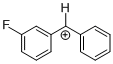 (mfp)PhCH+ | 0 | E Parameter: 6.23 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
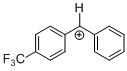 (tfm)PhCH+ | 0 | E Parameter: 6.70 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
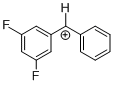 (dfp)PhCH+ | 0 | E Parameter: 6.74 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
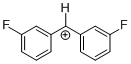 (mfp)2CH+ | 0 | E Parameter: 6.87 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
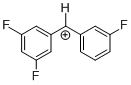 (dfp)(mfp)CH+ | 0 | E Parameter: 7.52 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
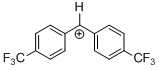 (tfm)2CH+ | 0 | E Parameter: 7.96 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
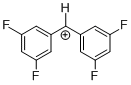 (dfp)2CH+ | 0 | E Parameter: 8.02 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
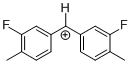 (Ftol)2CH+ | 0 | E Parameter: 5.24 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
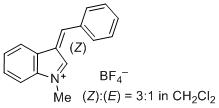 3-benzylidene-1-methyl-3H-indol-1-ium ion | 0 | E Parameter: -1.80 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
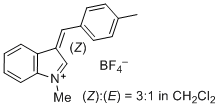 1-methyl-3-(4-methylbenzylidene)-3H-indol-1-ium ion | 0 | E Parameter: -2.19 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
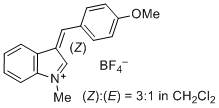 3-(4-methoxybenzylidene)-1-methyl-3H-indol-1-ium iom | 0 | E Parameter: -3.02 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
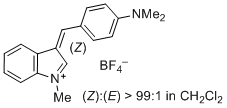 3-(4-(dimethylamino)benzylidene)-1-methyl-3H-indol-1-ium ion | 0 | E Parameter: -6.26 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
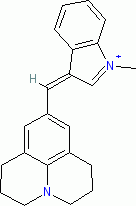 1-methyl-3-((julolidin-9-yl)methylene)-3H-indol-1-ium ion | 0 | E Parameter: -7.79 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
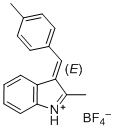 (E)-2-methyl-3-(4-methylbenzylidene)-3H-indol-1-ium ion | 0 | E Parameter: -5.15 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
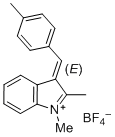 (E)-1,2-dimethyl-3-(4-methylbenzylidene)-3H-indol-1-ium ion | 0 | E Parameter: -5.05 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
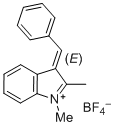 (E)-3-benzylidene-1,2-dimethyl-3H-indol-1-ium ion | 0 | E Parameter: -4.96 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
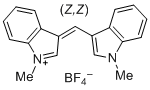 bis(1-methylindol-3-yl)methylium ion | 0 | E Parameter: -5.99 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
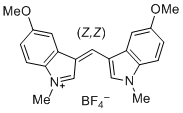 bis(5-methoxy-1-methylindol-3-yl)methylium ion | 0 | E Parameter: -6.90 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
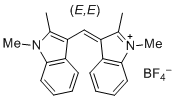 bis(1,2-dimethylindol-3-yl)methylium ion | 0 | E Parameter: -10.23 | J. Org. Chem. 2015, 80, 8643-8656 DOI: 10.1021/acs.joc.5b01298 | |
 Me2C=C=N(+)Me2 | 0 | E Parameter: -2.80 | Synthesis 2025, 57, 3251-3262 DOI: 10.1055/a-2649-1999 | |
 Ph2C=C=N(+)Me2 | 0 | E Parameter: -1.90 | Synthesis 2025, 57, 3251-3262 DOI: 10.1055/a-2649-1999 | |
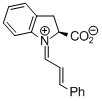 indolecarboxylate derived iminium ion | dichloromethane | E Parameter: -9.50 | Angew. Chem. Int. Ed. 2009, 48, 5034-5037 DOI: 10.1002/anie.200900933 | |
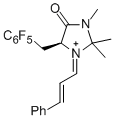 (S,E)-2,2,3-trimethyl-4-oxo-5-((perfluorophenyl)methyl)-1-((E)-3-phenylallylidene)imidazolidin-1-ium | dichloromethane | E Parameter: -6.00 | Angew. Chem. Int. Ed. 2013, 52, 7967-7971 DOI: 10.1002/anie.201301864 | |
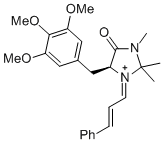 (S,E)-2,2,3-trimethyl-4-oxo-1-((E)-3-phenylallylidene)-5-(3,4,5-trimethoxybenzyl)imidazolidin-1-ium | dichloromethane | E Parameter: -7.00 | Angew. Chem. Int. Ed. 2013, 52, 7967-7971 DOI: 10.1002/anie.201301864 | |
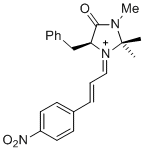 (S,E)-5-benzyl-2,2,3-trimethyl-1-((E)-3-(4-nitrophenyl)allylidene)-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -5.90 | Asian J. Org. Chem. 2014, 3, 550-555 DOI: 10.1002/ajoc.201402009 | |
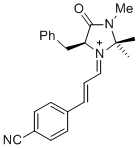 (S,E)-5-benzyl-1-((E)-3-(4-cyanophenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -6.00 | Asian J. Org. Chem. 2014, 3, 550-555 DOI: 10.1002/ajoc.201402009 | |
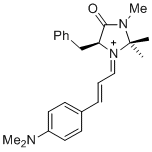 (S,E)-5-benzyl-1-((E)-3-(4-(dimethylamino)phenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -10.60 | Asian J. Org. Chem. 2014, 3, 550-555 DOI: 10.1002/ajoc.201402009 | |
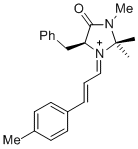 (S,E)-5-benzyl-2,2,3-trimethyl-4-oxo-1-((E)-3-(p-tolyl)allylidene)imidazolidin-1-ium | dichloromethane | E Parameter: -7.20 | Asian J. Org. Chem. 2014, 3, 550-555 DOI: 10.1002/ajoc.201402009 | |
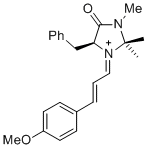 (S,E)-5-benzyl-1-((E)-3-(4-methoxyphenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -8.00 | Asian J. Org. Chem. 2014, 3, 550-555 DOI: 10.1002/ajoc.201402009 | |
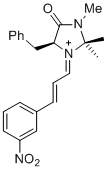 (S,E)-5-benzyl-2,2,3-trimethyl-1-((E)-3-(3-nitrophenyl)allylidene)-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -6.10 | Asian J. Org. Chem. 2014, 3, 550-555 DOI: 10.1002/ajoc.201402009 | |
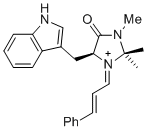 (S,E)-5-((1H-indol-3-yl)methyl)-2,2,3-trimethyl-4-oxo-1-((E)-3-phenylallylidene)imidazolidin-1-ium | dichloromethane | E Parameter: -7.30 | Asian J. Org. Chem. 2014, 3, 550-555 DOI: 10.1002/ajoc.201402009 | |
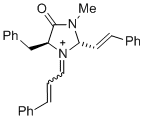 (2S,5S)-5-benzyl-3-methyl-4-oxo-1-(3-phenylallylidene)-2-((E)-styryl)imidazolidin-1-ium | dichloromethane | E Parameter: -5.90 | Asian J. Org. Chem. 2014, 3, 550-555 DOI: 10.1002/ajoc.201402009 | |
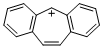 dibenzo[a,d]tropylium ion | dichloromethane | E Parameter: -0.63 | Liebigs Ann. 1995, , 2005-2009 DOI: 10.1002/jlac.1995199511281 | |
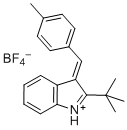 (E)-2-(tert-butyl)-3-(4-methylbenzylidene)-3H-indol-1-ium | dichloromethane | E Parameter: -5.06 | Eur. J. Org. Chem. 2016, , 4050-4058 DOI: 10.1002/ejoc.201600572 | |
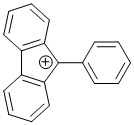 9-phenyl-9H-fluoren-9-ylium | dichloromethane | E Parameter: 2.41 | Chem. Eur. J. 2017, 23, 623-630 DOI: 10.1002/chem.201603963 | |
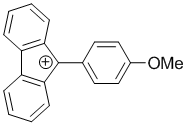 9-(4-methoxyphenyl)-9H-fluoren-9-ylium | dichloromethane | E Parameter: 0.85 | Chem. Eur. J. 2017, 23, 623-630 DOI: 10.1002/chem.201603963 | |
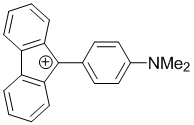 9-(4-(dimethylamino)phenyl)-9H-fluoren-9-ylium | dichloromethane | E Parameter: -4.51 | Chem. Eur. J. 2017, 23, 623-630 DOI: 10.1002/chem.201603963 | |
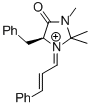 MacMillan-iminium ion (1st generation) | MeCN | E Parameter: -7.37 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 DOI: 10.1002/anie.201103683 | |
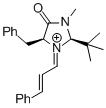 MacMillan-iminium ion (2nd generation) | MeCN | E Parameter: -5.52 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 DOI: 10.1002/anie.201103683 | |
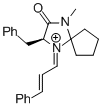 MacMillan-iminium ion (spiro) | MeCN | E Parameter: -7.67 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 DOI: 10.1002/anie.201103683 | |
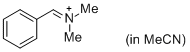 PhCH=N+Me2 (in MeCN) | MeCN | E Parameter: -9.27 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
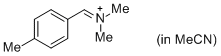 pTolCH=N+CH2 (in MeCN) | MeCN | E Parameter: -9.64 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
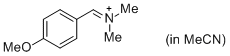 p-AniCH=N+Me2 (in MeCN) | MeCN | E Parameter: -10.69 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
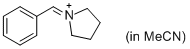 PhCH=N+(CH2)4 (in MeCN) | MeCN | E Parameter: -9.35 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
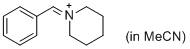 PhCH=N+(CH2)5 (in MeCN) | MeCN | E Parameter: -9.60 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
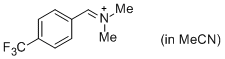 p-CF3-C6H4-CH=N+Me2 (in MeCN) | MeCN | E Parameter: -8.34 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x |