Nucleophiles
42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50
« Back Forward » Found 1335 molecules, displaying page 46 of 67 Results per page
10|50|100|all
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
3,5-dinitrobenzoate (in MeCN)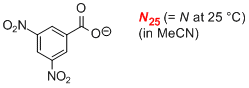  |
MeCN | N Param.: 14.90 sN Param.: 0.71 | J. Am. Chem. Soc. 2008, 130, 3012-3022 10.1021/ja0765464 | |
3,5-dinitrobenzoate (in acetone)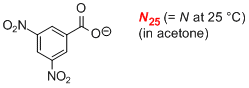  |
acetone | N Param.: 18.80 sN Param.: 0.62 | J. Am. Chem. Soc. 2008, 130, 3012-3022 10.1021/ja0765464 | |
2-methyl-pent-1-ene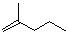  |
dichloromethane | N Param.: 0.84 sN Param.: 1.06 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | |
1-(N-piperidino)cyclohexene (in MeCN)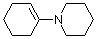  |
MeCN | N Param.: 14.02 sN Param.: 0.76 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
5-(trimethylsiloxy)-2,3-dihydrofuran (in MeCN)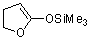  |
MeCN | N Param.: 12.34 sN Param.: 0.72 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
2-(trimethylsiloxy)-5,6-dihydro-4H-pyran (in MeCN)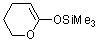  |
MeCN | N Param.: 10.52 sN Param.: 0.78 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
1-methoxy-2-methyl-1-(trimethylsiloxy)propene (in MeCN)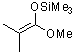  |
MeCN | N Param.: 9.11 sN Param.: 0.88 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
n-butyl vinyl ether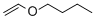  |
dichloromethane | N Param.: 3.76 sN Param.: 0.91 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | |
acetonitrile (in MeCN)  |
MeCN | N Param.: 2.23 sN Param.: 0.84 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | |
potassium trifluoro(1-methyl-1H-indol-2-yl)borate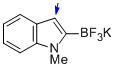  |
MeCN | N Param.: 9.55 sN Param.: 1.16 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
potassium trifluoro(1-methyl-1H-indol-5-yl)borate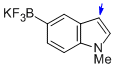  |
MeCN | N Param.: 8.77 sN Param.: 1.09 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
potassium (1-(tert-butoxycarbonyl)-1H-indol-2-yl)trifluoroborate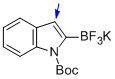  |
MeCN | N Param.: 6.46 sN Param.: 0.96 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
potassium (1-(tert-butoxycarbonyl)-5-methoxy-1H-indol-2-yl)trifluoroborate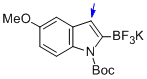  |
MeCN | N Param.: 7.10 sN Param.: 1.18 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
potassium (1-(tert-butoxycarbonyl)-1H-indol-3-yl)trifluoroborate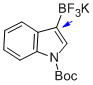  |
MeCN | N Param.: 4.06 sN Param.: 0.79 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
tert-butyl indole-1-carboxylate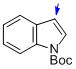  |
MeCN | N Param.: 1.68 sN Param.: 1.26 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
potassium trifluoro(furan-2-yl)borate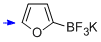  |
MeCN | N Param.: 5.99 sN Param.: 0.79 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
potassium trifluoro(furan-3-yl)borate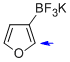  |
MeCN | N Param.: 6.83 sN Param.: 0.93 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
potassium trifluoro(3-methoxy-thiophen-2-yl)borate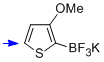  |
MeCN | N Param.: 7.32 sN Param.: 0.90 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
3-methoxythiophene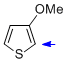  |
MeCN | N Param.: 3.06 sN Param.: 1.19 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
2-ethoxy-2-oxo-1-(pyridin-1-ium-1-yl)ethan-1-ide (in DMSO)  |
DMSO | N Param.: 26.71 sN Param.: 0.37 | J. Am. Chem. Soc. 2013, 135, 15216-15224 10.1021/ja407885h |
