Nucleophiles
55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63
« Back Forward » Found 1334 molecules, displaying page 59 of 67 Results per page
10|50|100|all
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
Ph3P=CH-CN  |
dichloromethane | N Param.: 12.29 sN Param.: 0.75 | J. Am. Chem. Soc. 2009, 131, 704-714 10.1021/ja8056216 | |
Ph3P=CH-CO2Et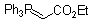  |
dichloromethane | N Param.: 12.79 sN Param.: 0.77 | J. Am. Chem. Soc. 2009, 131, 704-714 10.1021/ja8056216 | |
Ph3P=CH-CO2Et (in DMSO)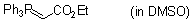  |
DMSO | N Param.: 12.21 sN Param.: 0.62 | J. Am. Chem. Soc. 2009, 131, 704-714 10.1021/ja8056216 | |
Ph3P=CH-COPh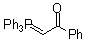  |
dichloromethane | N Param.: 9.54 sN Param.: 0.97 | J. Am. Chem. Soc. 2009, 131, 704-714 10.1021/ja8056216 | |
phenolate (in DMF)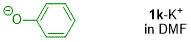  |
DMF | N Param.: 18.86 sN Param.: 0.89 | J. Org. Chem. 2019, 84, 8837-8858 10.1021/acs.joc.9b01485 | |
phenolate (in DMSO)  |
DMSO | N Param.: 19.86 sN Param.: 0.71 | J. Org. Chem. 2019, 84, 8837-8858 10.1021/acs.joc.9b01485 | |
phenolate (in MeCN)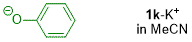  |
MeCN | N Param.: 18.53 sN Param.: 0.85 | J. Org. Chem. 2019, 84, 8837-8858 10.1021/acs.joc.9b01485 | |
phenylacetylene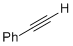  |
dichloromethane | N Param.: -0.04 sN Param.: 0.77 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 10.1002/anie.201402055 | |
phenylalanine (anionic, in water)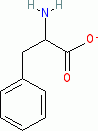  |
water | N Param.: 14.12 sN Param.: 0.53 | Org. Biomol. Chem. 2007, 5, 3814-3820 10.1039/b713778h | |
phenyldiazomethane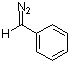  |
dichloromethane | N Param.: 9.35 sN Param.: 0.83 | Chem. Eur. J. 2003, 9, 4068-4076 10.1002/chem.200304913 | |
phenylsilane  |
dichloromethane | N Param.: 0.06 sN Param.: 0.71 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
phenylsulfinate (in 50W50AN)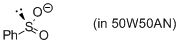  |
water-MeCN mix | N Param.: 13.75 sN Param.: 0.68 | J. Am. Chem. Soc. 2010, 132, 4796-4805 10.1021/ja9102056 | |
phenylsulfinate (in DMSO)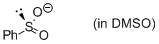  |
DMSO | N Param.: 19.60 sN Param.: 0.60 | J. Am. Chem. Soc. 2010, 132, 4796-4805 10.1021/ja9102056 | |
phenylsulfinate (in MeCN)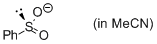  |
MeCN | N Param.: 20.11 sN Param.: 0.59 | J. Am. Chem. Soc. 2010, 132, 4796-4805 10.1021/ja9102056 | |
phthalimide anion (in DMSO)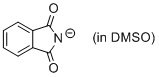  |
DMSO | N Param.: 15.52 sN Param.: 0.67 | J. Org. Chem. 2010, 75, 5250-5258 10.1021/jo1009883 | |
phthalimidoperoxyhexanoate (in H2O)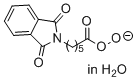  |
water | N Param.: 16.02 sN Param.: 0.54 | Angew. Chem. Int. Ed. 2017, 56, 13279-13282 10.1002/anie.201707086 | |
piperazine (in water)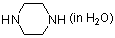  |
water | N Param.: 17.22 sN Param.: 0.50 | J. Org. Chem. 2007, 72, 3679-3688 10.1021/jo062586z | |
piperidin-1-yl dithiocarbamate (in MeCN)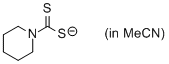  |
MeCN | N Param.: 23.84 sN Param.: 0.57 | Org. Biomol. Chem. 2011, 9, 8046-8050 10.1039/c1ob06245j | |
piperidine (in 91M9AN)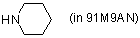  |
MeOH-MeCN mix | N Param.: 15.63 sN Param.: 0.64 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 10.1002/anie.200600542 | |
piperidine (in DMSO)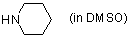  |
DMSO | N Param.: 17.19 sN Param.: 0.71 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y |
