Nucleophiles
59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67
« Back Forward » Found 1334 molecules, displaying page 65 of 67 Results per page
10|50|100|all
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
2-diazoindan-1-one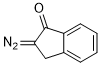  |
dichloromethane | N Param.: 5.61 sN Param.: 0.65 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazocyclohexanone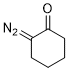  |
dichloromethane | N Param.: 3.44 sN Param.: 0.83 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-tetralone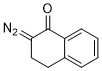  |
dichloromethane | N Param.: 3.51 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazoindandione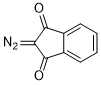  |
dichloromethane | N Param.: 0.16 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-benzosuberone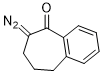  |
dichloromethane | N Param.: 2.72 sN Param.: 0.96 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazobenzothiophen-3(2H)-one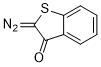  |
dichloromethane | N Param.: 0.40 sN Param.: 0.93 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in CH2Cl2)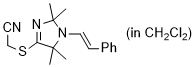  |
dichloromethane | N Param.: 7.06 sN Param.: 1.11 | Chem. Commun. 2023, 59, 8091-8084 10.1039/d3cc01912h | |
diazocyclopentadiene (in CH2Cl2)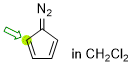  |
dichloromethane | N Param.: 4.84 sN Param.: 1.06 | Synthesis 2023, 55, 354-358 10.1055/s-0041-1737327 | |
(E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in CH2Cl2)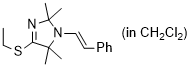  |
dichloromethane | N Param.: 8.13 sN Param.: 0.97 | Chem. Eur. J. 2024, 30, e202302764 10.1002/chem.202302764 | |
(E)-4-(benzylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in CH2Cl2)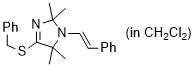  |
dichloromethane | N Param.: 7.99 sN Param.: 0.98 | Chem. Eur. J. 2024, 30, e202302764 10.1002/chem.202302764 | |
(F)2-HBTM (in CH2Cl2)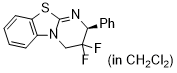  |
dichloromethane | N Param.: 10.83 sN Param.: 0.80 | ARKIVOC 2024, (4), 202312093 10.24820/ark.5550190[...] | |
2-methylpyridine (in CH2Cl2)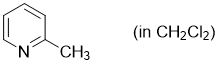  |
dichloromethane | N Param.: 10.52 sN Param.: 0.78 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,6-dimethylpyridine (in CH2Cl2)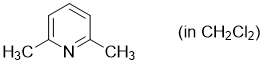  |
dichloromethane | N Param.: 9.87 sN Param.: 0.68 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,4,6-trimethylpyridine (in CH2Cl2)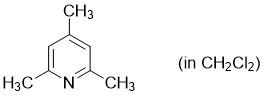  |
dichloromethane | N Param.: 9.34 sN Param.: 0.71 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
methyl diazoacetate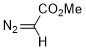  |
dichloromethane | N Param.: 4.68 sN Param.: 0.94 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
dimethyl diazomalonate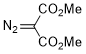  |
dichloromethane | N Param.: -1.24 sN Param.: 0.81 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Ph4P+) (in CH2Cl2)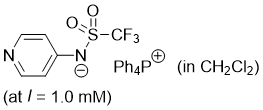  |
dichloromethane | N Param.: 17.88 sN Param.: 0.73 | J. Am. Chem. Soc. 2025, 147, 5043-5050 10.1021/jacs.4c14825 | |
((4-methoxyphenyl)sulfonyl)(pyridin-4-yl)amide (Ph4P+) (in CH2Cl2)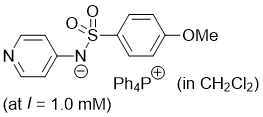  |
dichloromethane | N Param.: 19.63 sN Param.: 0.65 | J. Am. Chem. Soc. 2025, 147, 5043-5050 10.1021/jacs.4c14825 | |
O-DHPB (in CH2Cl2)  |
dichloromethane | N Param.: 13.31 sN Param.: 0.69 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
O-HyperBTM (in CH2Cl2)  |
dichloromethane | N Param.: 13.01 sN Param.: 0.73 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 |
