C-Nucleophiles
8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16
« Back Forward » Found 581 molecules, displaying page 12 of 30 Results per page
10|50|100|all
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
lithium 2-allyl-2-(3,5-bis(trifluoromethyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-uide (in MeCN)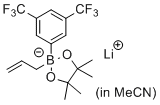  |
MeCN | N Param.: 8.71 sN Param.: 0.80 | J. Am. Chem. Soc. 2017, 139, 15324-15327 10.1021/jacs.7b10240 | |
lithium 2-allyl-4,4,5,5-tetramethyl-2-phenyl-1,3,2-dioxaborolan-2-uide (in MeCN)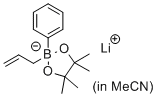  |
MeCN | N Param.: 11.20 sN Param.: 0.64 | J. Am. Chem. Soc. 2017, 139, 15324-15327 10.1021/jacs.7b10240 | |
lithium (E)-2-(3,5-bis(trifluoromethyl)phenyl)-2-(but-2-en-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-uide (in MeCN)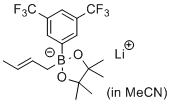  |
MeCN | N Param.: 9.49 sN Param.: 0.82 | J. Am. Chem. Soc. 2017, 139, 15324-15327 10.1021/jacs.7b10240 | |
(p-nitrophenyl)diazomethane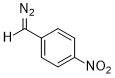  |
dichloromethane | N Param.: 7.17 sN Param.: 0.83 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
(p-cyanophenyl)diazomethane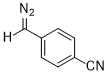  |
dichloromethane | N Param.: 7.66 sN Param.: 0.80 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
(p-bromophenyl)diazomethane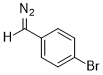  |
dichloromethane | N Param.: 8.87 sN Param.: 0.82 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
1-ethoxy-3-(4-nitrophenyl)-1,3-dioxopropan-2-ide (in DMSO)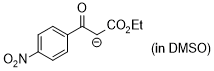  |
DMSO | N Param.: 16.26 sN Param.: 0.83 | J. Am. Chem. Soc. 2018, 140, 11474-11486 10.1021/jacs.8b07147 | |
Ph3P=CH-CHO (in MeCN)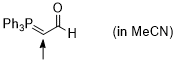  |
MeCN | N Param.: 9.09 sN Param.: 0.74 | J. Am. Chem. Soc. 2016, 138, 11272-11281 10.1021/jacs.6b06264 | |
Ph3P=CH-C(O)Me (in MeCN)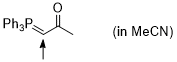  |
MeCN | N Param.: 10.27 sN Param.: 0.83 | J. Am. Chem. Soc. 2016, 138, 11272-11281 10.1021/jacs.6b06264 | |
anion of 4-(trifluoromethyl)benzyl trifluoromethyl sulfone (in MeCN)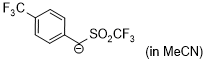  |
MeCN | N Param.: 16.15 sN Param.: 0.99 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
anion of 4-cyanobenzyl trifluoromethyl sulfone (in MeCN)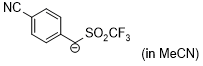  |
MeCN | N Param.: 15.62 sN Param.: 0.99 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
anion of 4-nitrobenzyl-CN (in MeCN)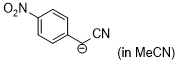  |
MeCN | N Param.: 20.10 sN Param.: 0.71 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
anion of (4-NO2-C6H4)CH2SO2Ph (in MeCN)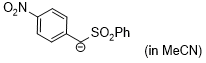  |
MeCN | N Param.: 19.90 sN Param.: 0.66 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
lithium 4,4,5,5-tetramethyl-2-phenyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolan-2-uide (in MeCN)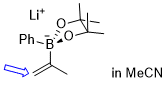  |
MeCN | N Param.: 11.00 sN Param.: 0.57 | J. Am. Chem. Soc. 2022, 144, 16118-16130 10.1021/jacs.2c06493 | |
methyl diazoacetate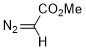  |
dichloromethane | N Param.: 4.68 sN Param.: 0.94 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
dimethyl diazomalonate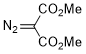  |
dichloromethane | N Param.: -1.24 sN Param.: 0.81 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
(allyl)dicarbonyl(cyclopentadienyl)iron(II)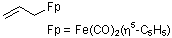  |
dichloromethane | N Param.: 6.78 sN Param.: 0.95 | Helv. Chim. Acta 2005, 88, 1754-1768 10.1002/hlca.200590137 | |
(2-methylallyl)dicarbonyl(cyclopentadienyl)iron(II)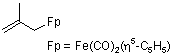  |
dichloromethane | N Param.: 8.45 sN Param.: 0.83 | Helv. Chim. Acta 2005, 88, 1754-1768 10.1002/hlca.200590137 | |
1,1-diethoxyethene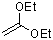  |
dichloromethane | N Param.: 9.81 sN Param.: 0.81 | Eur. J. Org. Chem. 2004, , 2791-2796 10.1002/ejoc.200400134 | |
1-butoxy-1-(trimethylsiloxy)ethene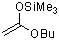  |
dichloromethane | N Param.: 10.21 sN Param.: 0.82 | Eur. J. Org. Chem. 2004, , 2791-2796 10.1002/ejoc.200400134 |
