Search
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
4-nitro-imidazole anion (in DMSO)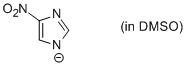  |
DMSO | N Param.: 14.81 sN Param.: 0.71 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
4-nitro-imidazole anion (in water)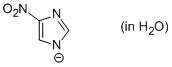  |
water | N Param.: 11.37 sN Param.: 0.53 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
4-nitrobenzodifuroxan (NBDF)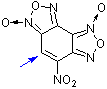  |
E Param.: -6.15 | Chem. Eur. J. 2007, 13, 8317-8324 10.1002/chem.200700676 | ||
4-nitroperoxybenzoate (in H2O)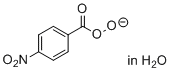  |
water | N Param.: 17.43 sN Param.: 0.50 | Angew. Chem. Int. Ed. 2017, 56, 13279-13282 10.1002/anie.201707086 | |
4-nitrothiophenolate (in DMSO)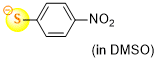  |
DMSO | N Param.: 18.92 sN Param.: 0.87 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
4-phenylbut-3-yn-2-one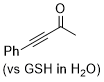  |
E Param.: -16.90 | Angew. Chem. Int. Ed. 2019, 58, 17704-17708 10.1002/anie.201909803 | ||
4-pyridone anion (in DMSO)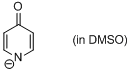  |
DMSO | N Param.: 18.97 sN Param.: 0.62 | J. Am. Chem. Soc. 2010, 132, 15380-15389 10.1021/ja106962u | |
4-pyridone anion (in MeCN)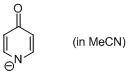  |
MeCN | N Param.: 20.22 sN Param.: 0.49 | J. Am. Chem. Soc. 2010, 132, 15380-15389 10.1021/ja106962u | |
4-pyridone anion (in water)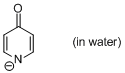  |
water | N Param.: 14.76 sN Param.: 0.48 | J. Am. Chem. Soc. 2010, 132, 15380-15389 10.1021/ja106962u | |
4-pyrrolidinopyridine (in CH2Cl2)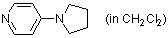  |
dichloromethane | N Param.: 15.90 sN Param.: 0.67 | Chem. Eur. J. 2007, 13, 336-345 10.1002/chem.200600941 | |
4-pyrrolidinopyridine (in H2O)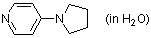  |
water | N Param.: 12.39 sN Param.: 0.66 | Chem. Eur. J. 2007, 13, 336-345 10.1002/chem.200600941 | |
4-pyrrolidinopyridine (in MeCN)  |
MeCN | N Param.: 14.99 sN Param.: 0.69 | Chem. Eur. J. 2013, 19, 6435-6442 10.1002/chem.201204452 | |
40% water/60%EtOH (v/v)  |
water-EtOH mix | N Param.: 6.28 sN Param.: 0.87 | J. Am. Chem. Soc. 2004, 126, 5174-5181 10.1021/ja031828z | |
40% water/60%TFE (v/v)  |
water-TFE mix | N Param.: 3.42 sN Param.: 0.90 | J. Am. Chem. Soc. 2004, 126, 5174-5181 10.1021/ja031828z | |
5% water/95% HFIP (w/w)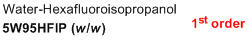  |
water-HFIP mix | N Param.: -0.10 sN Param.: 0.97 | J. Phys. Org. Chem. 2013, 26, 59-63 10.1002/poc.3064 | |
5,6-dimethyl-benzimidazole (in DMSO)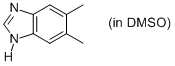  |
DMSO | N Param.: 11.08 sN Param.: 0.71 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
5,7,7-trichloro-8(7H)-quinolinone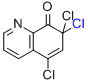  |
MeCN | E Param.: -10.48 | Org. Lett. 2010, 12, 2238-2241 10.1021/ol100592j | |
5-(4-(dimethylamino)benzyl)-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ide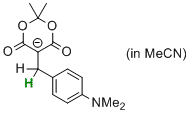  |
MeCN | N Param.: 7.15 sN Param.: 0.81 | Chem. Eur. J. 2014, 20, 11069-11077 10.1002/chem.201403161 | |
5-(4-(dimethylamino)benzyl)-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ide (in DMSO)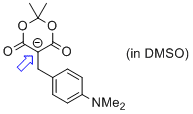  |
DMSO | N Param.: 14.48 sN Param.: 0.86 | Chem. Eur. J. 2014, 20, 11069-11077 10.1002/chem.201403161 | |
5-(4-(methoxy)benzyl)-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ide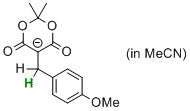  |
MeCN | N Param.: 6.21 sN Param.: 0.68 | Chem. Eur. J. 2014, 20, 11069-11077 10.1002/chem.201403161 |
News
- 01/30/26:
Isochalcogenoureas (O, S, Se & Te derivatives) have been added (Angew. Chem. Int. Ed. 2025, 64, e202514865). - 01/30/25:
Sesamol-derived ortho-quinone methides (o-QM) have been added (ChemEurJ 2024, e202403785 & OBC 2025, 23, 827-834) - 05/22/24:
Lactone enolates have been added (JOC 2024, 89, 6915-6928). - 11/13/24:
2-Methylene-1,2-dihydropyridines (2-pyNHOs) have been added (EurJOC 2024, 27, e202400373). - 05/21/24:
Mesoionic pyridinium-derived N-heterocyclic olefins (py-mNHOs) have been added (Angew. Chem. Int. Ed. 2024, 63, e202318283). - 10/19/23:
Enamines derived from 4-(alkylthio)-3-imidazolines and aldehydes have been added (ChemEurJ 2023, doi: 10.1002/chem.202302764). - 09/28/23:
Mesoionic N-heterocyclic olefins (mNHOs) have been added (Angew. Chem. Int. Ed. 2023, 62, e202309790). - 07/05/23:
S-Cyanomethylated imidazolidine-4-thione-derived enamines have been added (ChemComm 2023, 59, 8091). - 03/16/23:
Cyclic alpha-diazo carbonyl compounds added (EurJOC 2023, 26, e202300005) - 01/30/23:
NADH and NADPH reactivities (in water, pH 7) determined by Mayer and Moran have been added (OBC 2022, 21, 85-88). - 09/20/22:
Alkenyl boronate nucleophilicity by Liu, Ready and co-workers has been added (JACS 2022, 144, 16118). - 01/30/23:
Electrophilicities of diazo compounds in azo couplings (ChemEurJ 2022, 28, e202201376) - 01/30/23:
Diazocyclopentadiene added (Synthesis 2023, 55, 354-358) - 01/30/23:
Electrophilic indoles added (ChemCommun 2021, 57, 10071-10074) - 04/13/21:
Cyclic Michael acceptors added (Chem. Sci. 2021, 12, 4850-4865) - 05/21/21:
Thiophenolates (in DMSO) added (JOC 2021, 86, 5965-5972) - 05/06/20:
Heteroallenes added (JACS 2020, 142, 8383-8402). - 01/27/20:
Pyrrolidines and Imidazolidinones added (JACS 2020, 142, 1526-1547). - 05/06/20:
GSH reactivity added (Angew. Chem. Int. Ed. 2019, 58, 17704-17708) - 01/30/23:
Phenolates added (JOC 2019, 84, 8837-8858) - 04/24/18:
Ketones added (JACS 2018, 140, 5500). - 11/03/17:
Allyl-boron nucleophiles added (JACS 2017, 139, 15324) - 04/23/18:
Peroxide anions added (Angew. Chem. Int. Ed. 2017, 56, 13279). - 04/23/18:
Michael acceptors added (JACS 2017, 139, 13318). - 06/16/17:
Nucleophilicities of VNS reagents (JOC 2017, 82, 1011).
