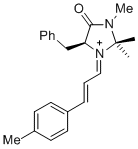
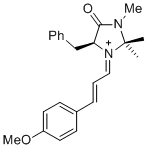
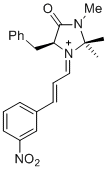
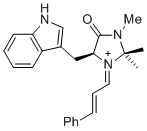
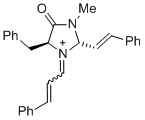
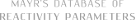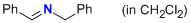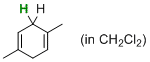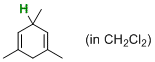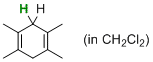| 1-phenyl-N-(phenylmethyl)methanimine (in CH2Cl2) |
dichloromethane | N Param.: 7.90 sN Param.: 0.76 |   | Z. Naturforsch. B 2013, 68b, 693-699
10.5560/ZNB.2013-3085 |
| (S,E)-2,2,3-trimethyl-4-oxo-5-((perfluorophenyl)methyl)-1-((E)-3-phenylallylidene)imidazolidin-1-ium |
dichloromethane | E Param.: -6.00 |   | Angew. Chem. Int. Ed. 2013, 52, 7967-7971
10.1002/anie.201301864 |
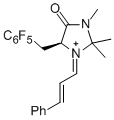
| (S,E)-2,2,3-trimethyl-4-oxo-1-((E)-3-phenylallylidene)-5-(3,4,5-trimethoxybenzyl)imidazolidin-1-ium |
dichloromethane | E Param.: -7.00 |   | Angew. Chem. Int. Ed. 2013, 52, 7967-7971
10.1002/anie.201301864 |
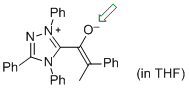
| (Z)-2-phenyl-1-(1,3,4-triphenyl-4H-1,2,4-triazol-1-ium-5-yl)prop-1-en-1-olate (in THF) |
THF | N Param.: 16.73 sN Param.: 0.63 |   | Angew. Chem. Int. Ed. 2013, 52, 11163-11167
10.1002/ange.201303524 |
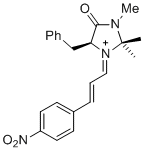
| (S,E)-5-benzyl-2,2,3-trimethyl-1-((E)-3-(4-nitrophenyl)allylidene)-4-oxoimidazolidin-1-ium |
dichloromethane | E Param.: -5.90 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
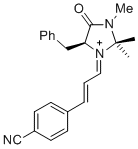
| (S,E)-5-benzyl-1-((E)-3-(4-cyanophenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium |
dichloromethane | E Param.: -6.00 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
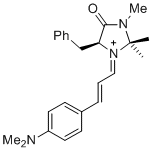
| (S,E)-5-benzyl-1-((E)-3-(4-(dimethylamino)phenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium |
dichloromethane | E Param.: -10.60 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
| (S,E)-5-benzyl-2,2,3-trimethyl-4-oxo-1-((E)-3-(p-tolyl)allylidene)imidazolidin-1-ium |
dichloromethane | E Param.: -7.20 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
| (S,E)-5-benzyl-1-((E)-3-(4-methoxyphenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium |
dichloromethane | E Param.: -8.00 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
| (S,E)-5-benzyl-2,2,3-trimethyl-1-((E)-3-(3-nitrophenyl)allylidene)-4-oxoimidazolidin-1-ium |
dichloromethane | E Param.: -6.10 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
| (S,E)-5-((1H-indol-3-yl)methyl)-2,2,3-trimethyl-4-oxo-1-((E)-3-phenylallylidene)imidazolidin-1-ium |
dichloromethane | E Param.: -7.30 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
| (2S,5S)-5-benzyl-3-methyl-4-oxo-1-(3-phenylallylidene)-2-((E)-styryl)imidazolidin-1-ium |
dichloromethane | E Param.: -5.90 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
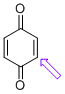
| p-quinone (C-H) |
| E Param.: -16.19 |   | J. Am. Chem. Soc. 2014, 136, 11499-11512
10.1021/ja505613b |
| 1,4-dimethylcyclohexa-1,4-diene (in CH2Cl2) |
dichloromethane | N Param.: 1.88 sN Param.: 0.96 |   | J. Am. Chem. Soc. 2014, 136, 13863-13873
10.1021/ja507598y |
| 1,3,5-trimethylcyclohexa-1,4-diene (in CH2Cl2) |
dichloromethane | N Param.: 4.95 sN Param.: 0.79 |   | J. Am. Chem. Soc. 2014, 136, 13863-13873
10.1021/ja507598y |
| 1,2,4,5-tetramethylcyclohexa-1,4-diene (in CH2Cl2) |
dichloromethane | N Param.: 4.27 sN Param.: 0.86 |   | J. Am. Chem. Soc. 2014, 136, 13863-13873
10.1021/ja507598y |
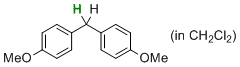
| bis(4-methoxyphenyl)methane (in CH2Cl2) |
dichloromethane | N Param.: -2.11 sN Param.: 0.98 |   | J. Am. Chem. Soc. 2014, 136, 13863-13873
10.1021/ja507598y |
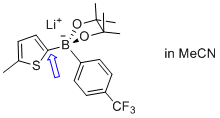
| lithium (5-methylthiophen-2-yl)(4-(trifluoromethyl)phenyl)pinacolborate |
MeCN | N Param.: 6.24 sN Param.: 1.00 |   | Angew. Chem. Int. Ed. 2015, 54, 2780-2783
10.1002/anie.201410562 |
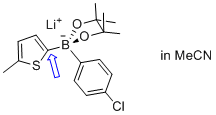
| lithium (5-methylthiophen-2-yl)(4-chlorophenyl)pinacolborate |
MeCN | N Param.: 6.77 sN Param.: 0.88 |   | Angew. Chem. Int. Ed. 2015, 54, 2780-2783
10.1002/anie.201410562 |
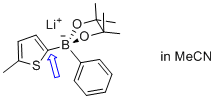
| lithium (5-methylthiophen-2-yl)(phenyl)pinacolborate |
MeCN | N Param.: 7.24 sN Param.: 0.83 |   | Angew. Chem. Int. Ed. 2015, 54, 2780-2783
10.1002/anie.201410562 |