Search
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
dimethyl((trimethylstannyl)methyl)silane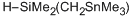  |
dichloromethane | N Param.: 6.53 sN Param.: 0.58 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
dimethyl((trimethylgermyl)methyl)silane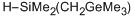  |
dichloromethane | N Param.: 5.36 sN Param.: 0.62 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
dimethyl diazomalonate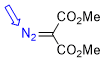  |
E Param.: -18.20 | Chem. Eur. J. 2022, 28, e202201376 10.1002/chem.202201376 | ||
dimethyl diazomalonate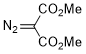  |
dichloromethane | N Param.: -1.24 sN Param.: 0.81 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
Dimethyl bis(indenyl)zirconium(IV)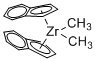  |
dichloromethane | N Param.: 6.89 sN Param.: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Dimethyl 1,1'-isopropylidenezirconocene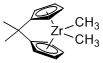  |
dichloromethane | N Param.: 5.20 sN Param.: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
dimethoxymethane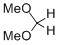  |
dichloromethane | N Param.: -4.90 sN Param.: 0.80 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
Dimedone iodonium ylide (in CH2Cl2)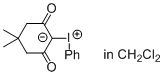  |
dichloromethane | N Param.: 6.18 sN Param.: 0.81 | J. Am. Chem. Soc. 2016, 138, 10304-10313 10.1021/jacs.6b05768 | |
diisopropyl azodicarboxylate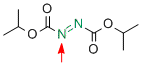  |
MeCN | E Param.: -10.71 | Chem. Eur. J. 2010, 16, 11670-11677 10.1002/chem.201001598 | |
dihydro-2H-pyran-2-one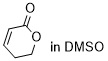  |
DMSO | E Param.: -21.80 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
dihexylsilane  |
dichloromethane | N Param.: 2.27 sN Param.: 0.73 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
diethylmercury (in MeCN)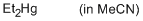  |
MeCN | N Param.: -0.70 sN Param.: 1.10 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
diethylether (in dichloromethane)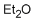  |
dichloromethane | N Param.: -5.10 sN Param.: 0.80 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
diethylamine (in water)  |
water | N Param.: 14.68 sN Param.: 0.53 | J. Org. Chem. 2007, 72, 3679-3688 10.1021/jo062586z | |
diethylamine (in MeCN)  |
MeCN | N Param.: 15.10 sN Param.: 0.73 | Eur. J. Org. Chem. 2009, , 6379-6385 10.1002/ejoc.200900925 | |
diethyl(methyl)silane  |
dichloromethane | N Param.: 3.40 sN Param.: 0.73 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
diethyl maleate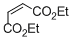  |
DMSO | E Param.: -19.49 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
diethyl fumarate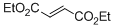  |
DMSO | E Param.: -17.79 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
diethyl diazomalonate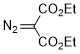  |
dichloromethane | N Param.: -0.35 sN Param.: 0.93 | Chem. Eur. J. 2003, 9, 4068-4076 10.1002/chem.200304913 | |
diethyl benzylidene malonate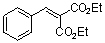  |
E Param.: -20.55 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 |
News
- 01/30/25:
Sesamol-derived ortho-quinone methides (o-QM) have been added (ChemEurJ 2024, e202403785 & OBC 2025, 23, 827-834) - 05/22/24:
Lactone enolates have been added (JOC 2024, 89, 6915-6928). - 11/13/24:
2-Methylene-1,2-dihydropyridines (2-pyNHOs) have been added (EurJOC 2024, 27, e202400373). - 05/21/24:
Mesoionic pyridinium-derived N-heterocyclic olefins (py-mNHOs) have been added (Angew. Chem. Int. Ed. 2024, 63, e202318283). - 10/19/23:
Enamines derived from 4-(alkylthio)-3-imidazolines and aldehydes have been added (ChemEurJ 2023, doi: 10.1002/chem.202302764). - 09/28/23:
Mesoionic N-heterocyclic olefins (mNHOs) have been added (Angew. Chem. Int. Ed. 2023, 62, e202309790). - 07/05/23:
S-Cyanomethylated imidazolidine-4-thione-derived enamines have been added (ChemComm 2023, 59, 8091). - 03/16/23:
Cyclic alpha-diazo carbonyl compounds added (EurJOC 2023, 26, e202300005) - 01/30/23:
NADH and NADPH reactivities (in water, pH 7) determined by Mayer and Moran have been added (OBC 2022, 21, 85-88). - 09/20/22:
Alkenyl boronate nucleophilicity by Liu, Ready and co-workers has been added (JACS 2022, 144, 16118). - 01/30/23:
Electrophilicities of diazo compounds in azo couplings (ChemEurJ 2022, 28, e202201376) - 01/30/23:
Diazocyclopentadiene added (Synthesis 2023, 55, 354-358) - 01/30/23:
Electrophilic indoles added (ChemCommun 2021, 57, 10071-10074) - 04/13/21:
Cyclic Michael acceptors added (Chem. Sci. 2021, 12, 4850-4865) - 05/21/21:
Thiophenolates (in DMSO) added (JOC 2021, 86, 5965-5972) - 05/06/20:
Heteroallenes added (JACS 2020, 142, 8383-8402). - 01/27/20:
Pyrrolidines and Imidazolidinones added (JACS 2020, 142, 1526-1547). - 05/06/20:
GSH reactivity added (Angew. Chem. Int. Ed. 2019, 58, 17704-17708) - 01/30/23:
Phenolates added (JOC 2019, 84, 8837-8858) - 04/24/18:
Ketones added (JACS 2018, 140, 5500). - 11/03/17:
Allyl-boron nucleophiles added (JACS 2017, 139, 15324) - 04/23/18:
Peroxide anions added (Angew. Chem. Int. Ed. 2017, 56, 13279). - 04/23/18:
Michael acceptors added (JACS 2017, 139, 13318). - 06/16/17:
Nucleophilicities of VNS reagents (JOC 2017, 82, 1011).
