N-Nucleophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
valine (anionic, in water)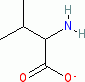  |
water | N Param.: 13.65 sN Param.: 0.57 | Org. Biomol. Chem. 2007, 5, 3814-3820 10.1039/b713778h | |
uracil anion (in water)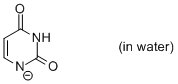  |
water | N Param.: 10.75 sN Param.: 0.53 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
uracil anion (in DMSO)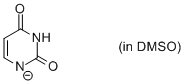  |
DMSO | N Param.: 17.04 sN Param.: 0.63 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
trimethylhydrazine (in MeCN)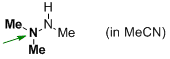  |
MeCN | N Param.: 17.75 sN Param.: 0.53 | Angew. Chem. Int. Ed. 2012, 51, 1353-1356 10.1002/anie.201107315 | |
trimethylhydrazine (in MeCN)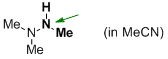  |
MeCN | N Param.: 12.43 sN Param.: 0.75 | Angew. Chem. Int. Ed. 2012, 51, 1353-1356 10.1002/anie.201107315 | |
trimethylamine (in MeCN)  |
MeCN | N Param.: 23.05 sN Param.: 0.45 | J. Org. Chem. 2012, 77, 8142-8155 10.1021/jo301497g | |
trifluoroacetamide anion (in DMSO)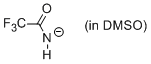  |
DMSO | N Param.: 15.81 sN Param.: 0.64 | J. Org. Chem. 2010, 75, 5250-5258 10.1021/jo1009883 | |
triethylamine (in MeCN)  |
MeCN | N Param.: 17.10 sN Param.: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 10.1002/poc.1707 | |
triethylamine (in CH2Cl2)  |
dichloromethane | N Param.: 17.30 sN Param.: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 10.1002/poc.1707 | |
thymine anion (in water)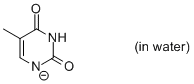  |
water | N Param.: 11.17 sN Param.: 0.51 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
thymine anion (in DMSO)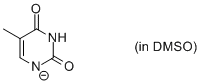  |
DMSO | N Param.: 17.63 sN Param.: 0.62 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
THTP (3,5,6,7-tetrahydro-2H-thiazolo[3,2-a]pyrimidine)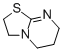  |
dichloromethane | N Param.: 14.45 sN Param.: 0.78 | J. Org. Chem. 2011, 76, 5104-5112 10.1021/jo200803x | |
threonine (anionic, in water)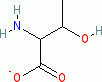  |
water | N Param.: 12.69 sN Param.: 0.60 | Org. Biomol. Chem. 2007, 5, 3814-3820 10.1039/b713778h | |
thiocyanate (in MeCN)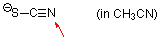  |
MeCN | N Param.: 12.13 sN Param.: 0.60 | J. Am. Chem. Soc. 2003, 125, 14126-14132 10.1021/ja037317u | |
theophylline anion (in water)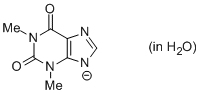  |
water | N Param.: 10.06 sN Param.: 0.71 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
theophylline anion (in DMSO)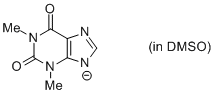  |
DMSO | N Param.: 14.78 sN Param.: 0.71 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
tetramisole (TM) (in CH2Cl2)  |
dichloromethane | N Param.: 13.86 sN Param.: 0.68 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
tert-butylamine (in water)  |
water | N Param.: 10.48 sN Param.: 0.65 | J. Org. Chem. 2007, 72, 3679-3688 10.1021/jo062586z | |
tert-butylamine (in MeCN)  |
MeCN | N Param.: 12.35 sN Param.: 0.72 | Eur. J. Org. Chem. 2009, , 6379-6385 10.1002/ejoc.200900925 | |
tert-butyl hydrazinecarboxylate (in MeCN)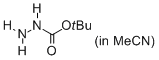  |
MeCN | N Param.: 11.40 sN Param.: 0.70 | J. Org. Chem. 2012, 77, 8142-8155 10.1021/jo301497g | |
Te-HyperBTM (in DCM)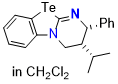  |
dichloromethane | N Param.: 16.63 sN Param.: 0.63 | ChemistryEurope 2026, 4, e202500443 10.1002/ceur.202500443 | |
Te-DHPB (in CH2Cl2)  |
dichloromethane | N Param.: 16.63 sN Param.: 0.65 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
Te-BTM (in CH2Cl2)  |
dichloromethane | N Param.: 14.62 sN Param.: 0.66 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
TCAP (in MeCN)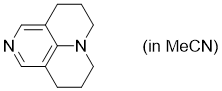  |
MeCN | N Param.: 15.60 sN Param.: 0.68 | Chem. Eur. J. 2013, 19, 6435-6442 10.1002/chem.201204452 | |
succinimide anion (in DMSO)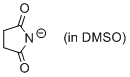  |
DMSO | N Param.: 16.03 sN Param.: 0.66 | J. Org. Chem. 2010, 75, 5250-5258 10.1021/jo1009883 | |
serine (anionic, in water)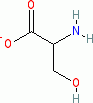  |
water | N Param.: 13.16 sN Param.: 0.55 | Org. Biomol. Chem. 2007, 5, 3814-3820 10.1039/b713778h | |
semicarbazide (in water)  |
water | N Param.: 11.05 sN Param.: 0.52 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | |
Se-tetramisole (Se-TM) (in CH2Cl2)  |
dichloromethane | N Param.: 15.26 sN Param.: 0.60 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
Se-HyperBTM (in THF)  |
THF | N Param.: 14.19 sN Param.: 0.77 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
Se-HyperBTM (in MeCN)  |
MeCN | N Param.: 15.84 sN Param.: 0.57 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
Se-HyperBTM (in DCM)  |
dichloromethane | N Param.: 16.11 sN Param.: 0.58 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
Se-HBTM (in CH2Cl2)  |
dichloromethane | N Param.: 14.42 sN Param.: 0.67 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
Se-DHPB (in CH2Cl2)  |
dichloromethane | N Param.: 15.16 sN Param.: 0.66 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
Se-BTM (in CH2Cl2)  |
dichloromethane | N Param.: 14.27 sN Param.: 0.63 | Angew. Chem. Int. Ed. 2025, 64, e202514865 10.1002/anie.202514865 | |
saccharin anion (in MeCN)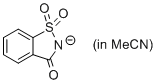  |
MeCN | N Param.: 10.78 sN Param.: 0.89 | J. Org. Chem. 2010, 75, 5250-5258 10.1021/jo1009883 | |
quinuclidine (in MeCN)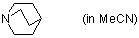  |
MeCN | N Param.: 20.54 sN Param.: 0.60 | Angew. Chem. Int. Ed. 2007, 46, 6176-6179 10.1002/anie.200701489 | |
quinine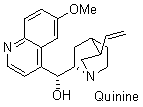  |
dichloromethane | N Param.: 10.46 sN Param.: 0.75 | J. Org. Chem. 2009, 74, 7157-7164 10.1021/jo901670w | |
quinidine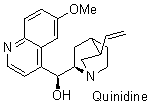  |
dichloromethane | N Param.: 10.54 sN Param.: 0.74 | J. Org. Chem. 2009, 74, 7157-7164 10.1021/jo901670w | |
pyrrolidine (inMeCN)  |
MeCN | N Param.: 18.58 sN Param.: 0.61 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
pyrrolidine (in water)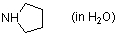  |
water | N Param.: 17.21 sN Param.: 0.49 | J. Org. Chem. 2007, 72, 3679-3688 10.1021/jo062586z | |
pyrrolidine (in 91M9AN)  |
MeOH-MeCN mix | N Param.: 15.97 sN Param.: 0.63 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 10.1002/anie.200600542 | |
pyridine (in MeCN)  |
MeCN | N Param.: 13.60 sN Param.: 0.60 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
pyridine (in H2O)  |
water | N Param.: 11.05 sN Param.: 0.73 | Chem. Eur. J. 2007, 13, 336-345 10.1002/chem.200600941 | |
pyridine (in CH2Cl2)  |
dichloromethane | N Param.: 12.90 sN Param.: 0.67 | Chem. Eur. J. 2007, 13, 336-345 10.1002/chem.200600941 | |
pyridin-4-yl(tosyl)amide (Ph4P+) (in MeCN)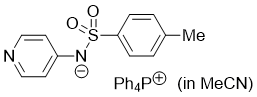  |
MeCN | N Param.: 17.19 sN Param.: 0.64 | J. Org. Chem. 2025, 90, 2298-2306 10.1021/acs.joc.4c02668 | |
pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Ph4P+) (in MeCN)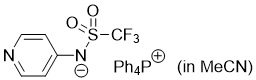  |
MeCN | N Param.: 16.38 sN Param.: 0.60 | J. Am. Chem. Soc. 2025, 147, 5043-5050 10.1021/jacs.4c14825 | |
pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Ph4P+) (in CH2Cl2)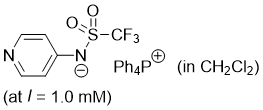  |
dichloromethane | N Param.: 17.88 sN Param.: 0.73 | J. Am. Chem. Soc. 2025, 147, 5043-5050 10.1021/jacs.4c14825 | |
pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Bu4P+) (in MeCN)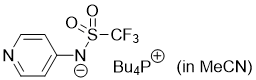  |
MeCN | N Param.: 16.48 sN Param.: 0.60 | J. Org. Chem. 2025, 90, 2298-2306 10.1021/acs.joc.4c02668 | |
pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Bu4N+) (in MeCN)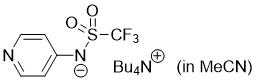  |
MeCN | N Param.: 16.68 sN Param.: 0.59 | J. Org. Chem. 2025, 90, 2298-2306 10.1021/acs.joc.4c02668 | |
pyridin-4-yl((4-(trifluoromethyl)phenyl)sulfonyl)amide (Ph4P+) (in MeCN)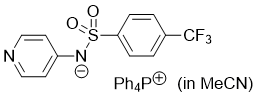  |
MeCN | N Param.: 19.51 sN Param.: 0.47 | J. Org. Chem. 2025, 90, 2298-2306 10.1021/acs.joc.4c02668 |
