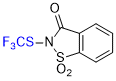
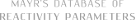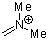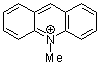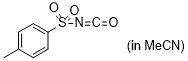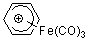| N-(trifluoromethyl)thio)saccharin |
| E Param.: -6.48 |    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
10.1002/anie.201805859 |
| dimethylmethyleneammonium ion |
| E Param.: -6.69 |  | Acc. Chem. Res. 2003, 36, 66-77
10.1021/ar020094c |
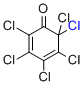
| 2,3,4,5,6,6-hexachlorocyclohexa-2,4-dien-1-one |
MeCN | E Param.: -6.75 |    | Org. Lett. 2010, 12, 2238-2241
10.1021/ol100592j |
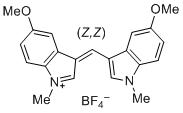
| bis(5-methoxy-1-methylindol-3-yl)methylium ion |
| E Param.: -6.90 |    | J. Org. Chem. 2015, 80, 8643-8656
10.1021/acs.joc.5b01298 |
| (S,E)-2,2,3-trimethyl-4-oxo-1-((E)-3-phenylallylidene)-5-(3,4,5-trimethoxybenzyl)imidazolidin-1-ium |
dichloromethane | E Param.: -7.00 |   | Angew. Chem. Int. Ed. 2013, 52, 7967-7971
10.1002/anie.201301864 |
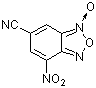
| 6-cyano-4-nitrobenzofuroxan |
| E Param.: -7.01 |    | J. Org. Chem. 2005, 70, 6242-6253
10.1021/jo0505526 |
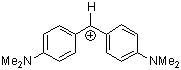
| (dma)2CH+ |
| E Param.: -7.02 |      | J. Am. Chem. Soc. 2001, 123, 9500-9512
10.1021/ja010890y |
| N-methylacridinium ion |
| E Param.: -7.15 |    | Acc. Chem. Res. 2003, 36, 66-77
10.1021/ar020094c |
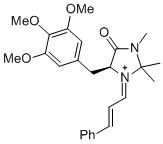
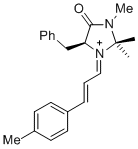
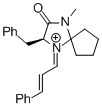
| (S,E)-5-benzyl-2,2,3-trimethyl-4-oxo-1-((E)-3-(p-tolyl)allylidene)imidazolidin-1-ium |
dichloromethane | E Param.: -7.20 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
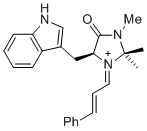
| (S,E)-5-((1H-indol-3-yl)methyl)-2,2,3-trimethyl-4-oxo-1-((E)-3-phenylallylidene)imidazolidin-1-ium |
dichloromethane | E Param.: -7.30 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |
| MacMillan-iminium ion (1st generation) |
MeCN | E Param.: -7.37 |    | Angew. Chem. Int. Ed. 2011, 50, 9953-9956
10.1002/anie.201103683 |
| diarylallylium ion (4-NMe2)2 |
| E Param.: -7.50 |    | J. Org. Chem. 2011, 76, 9391-9408
10.1021/jo201668w |
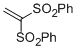
| (ethene-1,1-diyldisulfonyl)dibenzene |
DMSO | E Param.: -7.50 |    | Chem. Asian J. 2012, 7, 1401-1407
10.1002/asia.201101046 |
| MacMillan-iminium ion (spiro) |
MeCN | E Param.: -7.67 |    | Angew. Chem. Int. Ed. 2011, 50, 9953-9956
10.1002/anie.201103683 |
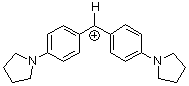
| (pyr)2CH+ |
| E Param.: -7.69 |      | J. Am. Chem. Soc. 2001, 123, 9500-9512
10.1021/ja010890y |
| p-tosyl isocyanate |
MeCN | E Param.: -7.69 |    | J. Am. Chem. Soc. 2020, 142, 8383-8402
10.1021/jacs.0c01960 |
| C6H7+-Fe(CO)3 |
| E Param.: -7.76 |    | Acc. Chem. Res. 2003, 36, 66-77
10.1021/ar020094c |
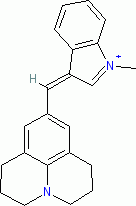
| 1-methyl-3-((julolidin-9-yl)methylene)-3H-indol-1-ium ion |
| E Param.: -7.79 |    | J. Org. Chem. 2015, 80, 8643-8656
10.1021/acs.joc.5b01298 |
| (4-NMe2)-tritylium ion |
| E Param.: -7.93 |  | J. Am. Chem. Soc. 2003, 125, 286-295
10.1021/ja021010y |
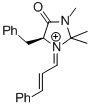
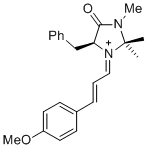
| (S,E)-5-benzyl-1-((E)-3-(4-methoxyphenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium |
dichloromethane | E Param.: -8.00 |   | Asian J. Org. Chem. 2014, 3, 550-555
10.1002/ajoc.201402009 |