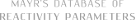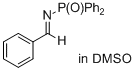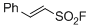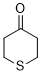| (E)-N-benzylidene-P,P-diphenylphosphinic amide |
DMSO | E Param.: -15.89 |    | J. Am. Chem. Soc. 2011, 133, 8240-8251
10.1021/ja200820m |
| p-nitrophenyl isothiocyanate |
DMSO | E Param.: -15.89 |    | J. Am. Chem. Soc. 2020, 142, 8383-8402
10.1021/jacs.0c01960 |
| (E)-10-(4-methoxybenzylidene)phenanthren-9(10H)-one |
| E Param.: -15.93 |    | J. Phys. Org. Chem. 2026, 39, e70061
10.1002/poc.70061 |
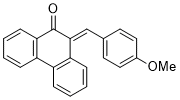
| (E)-6-((E)-3-phenylallylidene)benzo[d][1,3]dioxol-5(6H)-one |
| E Param.: -16.00 |    | Org. Biomol. Chem. 2025, 23, 827-834
10.1039/D4OB01855A |
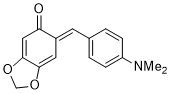
| (E)-6-(4-(dimethylamino)benzylidene)benzo[d][1,3]dioxol-5(6H)-one |
| E Param.: -16.07 |    | Chem. Eur. J. 2025, 31, e202403785
10.1002/chem.202403785 |
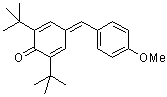
| ani(t-Bu)2QM |
| E Param.: -16.11 |     | Angew. Chem. Int. Ed. 2002, 41, 91-95
10.1002/1521-3773(20[...] |
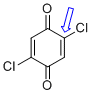
| 2,5-dichloroquinone (C-Cl) |
| E Param.: -16.11 |    | J. Am. Chem. Soc. 2014, 136, 11499-11512
10.1021/ja505613b |
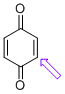
| p-quinone (C-H) |
| E Param.: -16.19 |   | J. Am. Chem. Soc. 2014, 136, 11499-11512
10.1021/ja505613b |
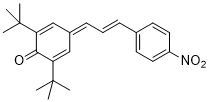
| (E)-2,6-di-tert-butyl-4-(3-(4-nitrophenyl)allylidene)cyclohexa-2,5-dien-1-one |
DMSO | E Param.: -16.25 |    | Org. Lett. 2020, 22, 2182-2186
10.1021/acs.orglett.[...] |
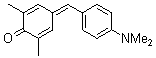
| 2,6-dimethyl-4-(4-(dimethylamino)benzylidene)cyclohexa-2,5-dienone |
| E Param.: -16.36 |    | Eur. J. Org. Chem. 2009, , 3203-3211
10.1002/ejoc.200900299 |
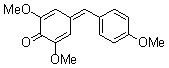
| 2,6-dimethoxy-4-(4-methoxybenzylidene)cyclohexa-2,5-dienone |
| E Param.: -16.38 |    | Eur. J. Org. Chem. 2009, , 3203-3211
10.1002/ejoc.200900299 |
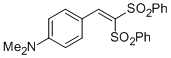
| 4-(2,2-bis(phenylsulfonyl)vinyl)-N,N-dimethylaniline |
DMSO | E Param.: -16.53 |    | Chem. Asian J. 2012, 7, 1401-1407
10.1002/asia.201101046 |
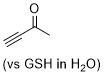
| butynone |
| E Param.: -16.60 |  | Angew. Chem. Int. Ed. 2019, 58, 17704-17708
10.1002/anie.201909803 |
| 2-phenylethene-1-sulfonyl fluoride |
DMSO | E Param.: -16.63 |    | Angew. Chem. Int. Ed. 2016, 55, 12664-12667
10.1002/anie.201601875 |
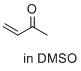
| but-3-en-2-one (in DMSO) |
DMSO | E Param.: -16.76 |    | J. Am. Chem. Soc. 2017, 139, 13318-13329
10.1021/jacs.7b05106 |
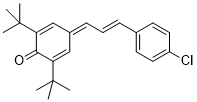
| E)-2,6-di-tert-butyl-4-(3-(4-chlorophenyl)allylidene)cyclohexa-2,5-dien-1-one |
DMSO | E Param.: -16.84 |    | Org. Lett. 2020, 22, 2182-2186
10.1021/acs.orglett.[...] |
| thian-4-one (in DMSO) |
DMSO | E Param.: -16.90 |  | J. Am. Chem. Soc. 2018, 140, 5500-5515
10.1021/jacs.8b01657 |
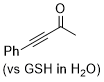
| 4-phenylbut-3-yn-2-one |
| E Param.: -16.90 |  | Angew. Chem. Int. Ed. 2019, 58, 17704-17708
10.1002/anie.201909803 |
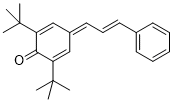
| (E)-2,6-di-tert-butyl-4-(3-phenylallylidene)cyclohexa-2,5-dien-1-one |
DMSO | E Param.: -17.00 |    | Org. Lett. 2020, 22, 2182-2186
10.1021/acs.orglett.[...] |
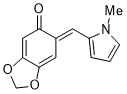
| (E)-6-((1-methyl-1H-pyrrol-2-yl)methylene)benzo[d][1,3]dioxol-5(6H)-one |
| E Param.: -17.03 |    | Org. Biomol. Chem. 2025, 23, 827-834
10.1039/D4OB01855A |