Nucleophiles
24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32
« Back Forward » Found 1334 molecules, displaying page 28 of 67 Results per page
10|50|100|all
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
(S)-N-propylpyrrolidine-2-carboxamide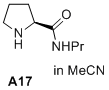  |
MeCN | N Param.: 15.20 sN Param.: 0.73 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-2-(pyrrolidin-2-ylmethyl)isoindoline-1,3-dione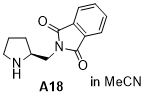  |
MeCN | N Param.: 15.90 sN Param.: 0.77 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-4-phenyl-1-(pyrrolidin-2-ylmethyl)-1H-1,2,3-triazole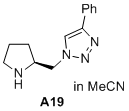  |
MeCN | N Param.: 15.32 sN Param.: 0.72 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-1-(pyrrolidin-2-ylmethyl)-1H-imidazole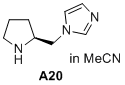  |
MeCN | N Param.: 15.55 sN Param.: 0.69 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-3-butyl-1-(pyrrolidin-2-ylmethyl)-1H-imidazol-3-ium trifluoromethanesulfonate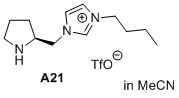  |
MeCN | N Param.: 13.57 sN Param.: 0.53 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-1-(3,5-bis(trifluoromethyl)phenyl)-3-(pyrrolidin-2-ylmethyl)thiourea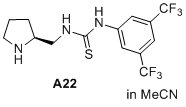  |
MeCN | N Param.: 14.97 sN Param.: 0.69 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-1-(3,5-bis(trifluoromethyl)phenyl)-3-(pyrrolidin-2-ylmethyl)urea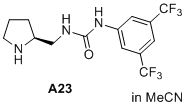  |
MeCN | N Param.: 17.50 sN Param.: 0.64 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
2-tritylpyrrolidine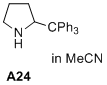  |
MeCN | N Param.: 9.16 sN Param.: 1.39 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-2-(diphenyl((trimethylsilyl)oxy)methyl)pyrrolidine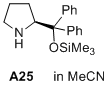  |
MeCN | N Param.: 12.03 sN Param.: 0.98 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-diphenyl(pyrrolidin-2-yl)methanol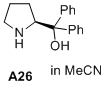  |
MeCN | N Param.: 16.18 sN Param.: 0.56 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-2-(azidodiphenylmethyl)pyrrolidine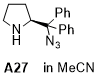  |
MeCN | N Param.: 9.90 sN Param.: 1.22 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
2-(triphenylsilyl)pyrrolidine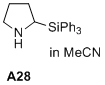  |
MeCN | N Param.: 14.00 sN Param.: 0.84 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(S)-5-benzyl-2,2,3-trimethylimidazolidin-4-one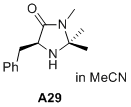  |
MeCN | N Param.: 6.04 sN Param.: 0.92 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(2S,5S)-5-benzyl-2-(tert-butyl)-3-methylimidazolidin-4-one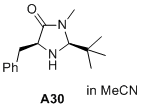  |
MeCN | N Param.: 5.44 sN Param.: 1.12 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(2S,5S)-5-benzyl-3-methyl-2-(5-methylfuran-2-yl)imidazolidin-4-one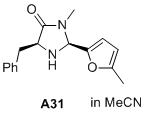  |
MeCN | N Param.: 8.76 sN Param.: 0.89 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(2R,5S)-5-benzyl-3-methyl-2-(5-methylfuran-2-yl)imidazolidin-4-one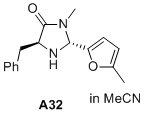  |
MeCN | N Param.: 7.39 sN Param.: 1.00 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
dimethylsulfide (in MeCN)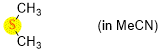  |
MeCN | N Param.: 12.70 sN Param.: 0.72 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
TCAP (in MeCN)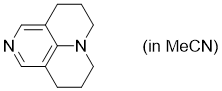  |
MeCN | N Param.: 15.60 sN Param.: 0.68 | Chem. Eur. J. 2013, 19, 6435-6442 10.1002/chem.201204452 | |
tetrahydrothiophene (in MeCN)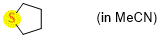  |
MeCN | N Param.: 13.30 sN Param.: 0.72 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
anion of 4-(trifluoromethyl)benzyl trifluoromethyl sulfone (in MeCN)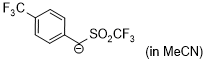  |
MeCN | N Param.: 16.15 sN Param.: 0.99 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 |
