Nucleophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
1-ethinylcyclohexenyl-Co2(CO)6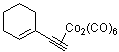  |
dichloromethane | N Param.: -0.44 sN Param.: 1.06 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
1-ethoxy-2-methyl-1,3-dioxobutan-2-ide (in DMSO)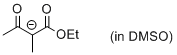  |
DMSO | N Param.: 19.58 sN Param.: 0.73 | Eur. J. Org. Chem. 2015, , 7594-7601 10.1002/ejoc.201501107 | |
1-ethoxy-3-(4-nitrophenyl)-1,3-dioxopropan-2-ide (in DMSO)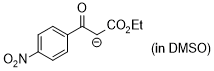  |
DMSO | N Param.: 16.26 sN Param.: 0.83 | J. Am. Chem. Soc. 2018, 140, 11474-11486 10.1021/jacs.8b07147 | |
1-hexene  |
dichloromethane | N Param.: -2.77 sN Param.: 1.41 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | |
1-mesityl-3-methyl-5-methylene-2,4,6-triphenyl-2,5-dihydropyridin-1-ium-2-ide (in THF)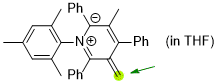  |
THF | N Param.: 26.16 sN Param.: 0.52 | Angew. Chem. Int. Ed. 2024, , e202318283 10.1002/anie.202318283 | |
1-methoxy-2-methyl-1-(trimethylsiloxy)propene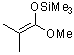  |
dichloromethane | N Param.: 9.00 sN Param.: 0.98 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
1-methoxy-2-methyl-1-(trimethylsiloxy)propene (in MeCN)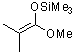  |
MeCN | N Param.: 9.11 sN Param.: 0.88 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
1-methyl uracil anion (in DMSO)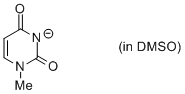  |
DMSO | N Param.: 16.36 sN Param.: 0.69 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
1-methyl uracil anion (in water)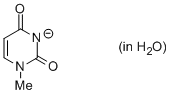  |
water | N Param.: 8.54 sN Param.: 0.77 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
1-methyl-2,3,4,6,7,8-hexahydro-1H-pyrimido[1,2-a]pyrimidine (MeTBD)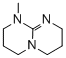  |
dichloromethane | N Param.: 14.43 sN Param.: 0.81 | ChemCatChem 2012, 4, 993-999 10.1002/cctc.201200143 | |
1-methyl-2-methylene-3-phenyl-1,2-dihydropyridine (in DMSO)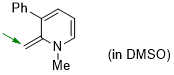  |
DMSO | N Param.: 19.41 sN Param.: 0.61 | Eur. J. Org. Chem. 2024, 27, e202400373 10.1002/ejoc.202400373 | |
1-methyl-2-phenylpyrrolidine (in CH2Cl2)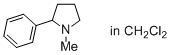  |
dichloromethane | N Param.: 16.80 sN Param.: 0.49 | J. Phys. Org. Chem. 2016, 29, 759-767 10.1002/poc.3580 | |
1-methyl-2-phenylpyrrolidine (in MeCN)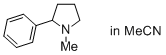  |
MeCN | N Param.: 15.70 sN Param.: 0.54 | J. Phys. Org. Chem. 2016, 29, 759-767 10.1002/poc.3580 | |
1-methyl-4-(N-morpholino)tetrahydropyridine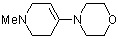  |
dichloromethane | N Param.: 12.03 sN Param.: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 10.1002/chem.200204666 | |
1-methyl-4-vinyl-benzene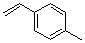  |
dichloromethane | N Param.: 1.70 sN Param.: 1.06 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
1-methyl-benzimidazole (in MeCN)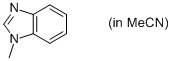  |
MeCN | N Param.: 10.37 sN Param.: 0.82 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
1-methyl-benzotriazole (in MeCN)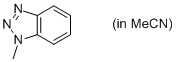  |
MeCN | N Param.: 7.77 sN Param.: 0.76 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
1-methyl-imidazole (in MeCN)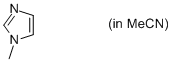  |
MeCN | N Param.: 11.90 sN Param.: 0.73 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
1-methyl-imidazole (in water)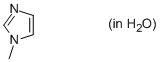  |
water | N Param.: 9.91 sN Param.: 0.55 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
1-methylcyclohexene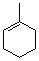  |
dichloromethane | N Param.: 0.08 sN Param.: 1.15 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
1-methylcyclopentene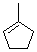  |
dichloromethane | N Param.: 1.18 sN Param.: 1.17 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | |
1-methylsiletane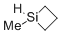  |
dichloromethane | N Param.: 2.30 sN Param.: 0.75 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
1-methylsilolane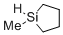  |
dichloromethane | N Param.: 2.20 sN Param.: 0.75 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
1-phenoxy-1-(trimethylsiloxy)ethene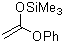  |
dichloromethane | N Param.: 8.23 sN Param.: 0.81 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
1-phenyl-1-(trimethylsiloxy)ethene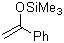  |
dichloromethane | N Param.: 6.22 sN Param.: 0.96 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
1-phenyl-imidazole (in MeCN)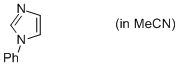  |
MeCN | N Param.: 11.31 sN Param.: 0.67 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
1-phenyl-N-(phenylmethyl)methanimine (in CH2Cl2)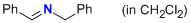  |
dichloromethane | N Param.: 7.90 sN Param.: 0.76 | Z. Naturforsch. B 2013, 68b, 693-699 10.5560/ZNB.2013-3085 | |
1-pyrrolidino-3,4-dihydronaphthalene (in MeCN)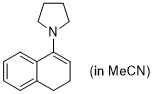  |
MeCN | N Param.: 14.09 sN Param.: 0.66 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
10% ethanol/90% MeCN (v/v)  |
EtOH-MeCN mix | N Param.: 5.19 sN Param.: 0.96 | J. Am. Chem. Soc. 2004, 126, 5174-5181 10.1021/ja031828z | |
10% methanol/90% MeCN (v/v)  |
MeOH-MeCN mix | N Param.: 5.55 sN Param.: 0.97 | J. Am. Chem. Soc. 2004, 126, 5174-5181 10.1021/ja031828z | |
10% water/90% acetone (v/v)  |
water-acetone mix | N Param.: 5.70 sN Param.: 0.85 | Angew. Chem. Int. Ed. 2004, 43, 2302-2305 10.1002/anie.200353468 | |
10% water/90% HFIP (w/w)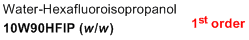  |
water-HFIP mix | N Param.: 0.96 sN Param.: 0.93 | J. Phys. Org. Chem. 2013, 26, 59-63 10.1002/poc.3064 | |
10% water/90% MeCN (v/v)  |
water-MeCN mix | N Param.: 4.56 sN Param.: 0.94 | J. Am. Chem. Soc. 2004, 126, 5174-5181 10.1021/ja031828z | |
10% water/90%EtOH (v/v)  |
water-EtOH mix | N Param.: 7.03 sN Param.: 0.86 | J. Am. Chem. Soc. 2004, 126, 5174-5181 10.1021/ja031828z | |
10% water/90%TFE (v/v)  |
water-TFE mix | N Param.: 2.93 sN Param.: 0.88 | J. Am. Chem. Soc. 2004, 126, 5174-5181 10.1021/ja031828z | |
10-methyl-9,10-dihydroacridine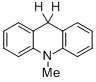  |
dichloromethane | N Param.: 5.54 sN Param.: 0.90 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
10-methyl-9,10-dihydroacridine (in MeCN)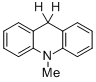  |
MeCN | N Param.: 4.00 sN Param.: 0.70 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
1H-indole-5-carboxylic acid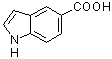  |
MeCN | N Param.: 3.97 sN Param.: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 10.1021/jo0614339 | |
2% water/98% HFIP (w/w)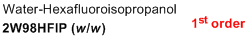  |
water-HFIP mix | N Param.: -1.62 sN Param.: 1.10 | J. Phys. Org. Chem. 2013, 26, 59-63 10.1002/poc.3064 | |
2,2,2-trifluoroethanolate (in water)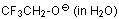  |
water | N Param.: 12.66 sN Param.: 0.59 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | |
2,2,2-trifluoroethylamine (in 91M9AN)  |
MeOH-MeCN mix | N Param.: 10.20 sN Param.: 0.92 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 10.1002/anie.200600542 | |
2,2,2-trifluoroethylamine (in DMSO)  |
DMSO | N Param.: 12.15 sN Param.: 0.65 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | |
2,2,2-trifluoroethylamine (in MeCN)  |
MeCN | N Param.: 10.13 sN Param.: 0.75 | Eur. J. Org. Chem. 2009, , 6379-6385 10.1002/ejoc.200900925 | |
2,2-dimethyl-5-(4-nitrobenzyl)-4,6-dioxo-1,3-dioxan-5-ide (in DMSO)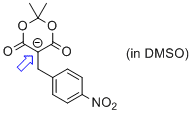  |
DMSO | N Param.: 12.02 sN Param.: 1.17 | Chem. Eur. J. 2014, 20, 11069-11077 10.1002/chem.201403161 | |
2,2-dimethylpyrrolidine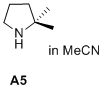  |
MeCN | N Param.: 13.96 sN Param.: 0.76 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
2,3,3-trimethyl-but-1-ene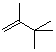  |
dichloromethane | N Param.: 0.06 sN Param.: 1.07 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | |
2,3,4,6,7,8-hexahydro-1H-pyrimido[1,2-a]pyrimidine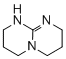  |
dichloromethane | N Param.: 16.16 sN Param.: 0.75 | ChemCatChem 2012, 4, 993-999 10.1002/cctc.201200143 | |
2,3,4,6,7,8-hexahydropyrimido[2,1-b][1,3]thiazine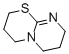  |
dichloromethane | N Param.: 14.10 sN Param.: 0.82 | J. Org. Chem. 2011, 76, 5104-5112 10.1021/jo200803x | |
2,3,5,6-tetrahydro-1H-imidazo[1,2-a]imidazole (TBO)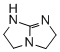  |
dichloromethane | N Param.: 14.44 sN Param.: 0.79 | ChemCatChem 2012, 4, 993-999 10.1002/cctc.201200143 | |
2,3,5,6-tetrahydroimidazo[2,1-b]thiazole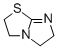  |
dichloromethane | N Param.: 12.98 sN Param.: 0.81 | J. Org. Chem. 2011, 76, 5104-5112 10.1021/jo200803x |
