C-Nucleophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
lithium (5-methylthiophen-2-yl)(4-methoxyphenyl)pinacolborate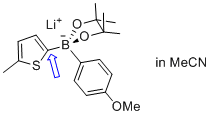  |
MeCN | N Param.: 7.51 sN Param.: 0.87 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
lithium (5-methylthiophen-2-yl)(4-chlorophenyl)pinacolborate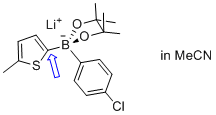  |
MeCN | N Param.: 6.77 sN Param.: 0.88 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
lithium (5-methylthiophen-2-yl)(4-(trifluoromethyl)phenyl)neopentylglycolborate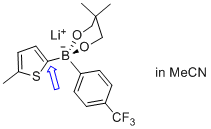  |
MeCN | N Param.: 11.85 sN Param.: 0.72 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
lithium (5-methylthiophen-2-yl)(4-(trifluoromethyl)phenyl)catecholglycolborate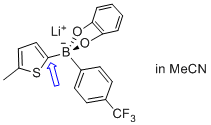  |
MeCN | N Param.: 6.50 sN Param.: 0.77 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
lithium (5-methylthiophen-2-yl)(4-(dimethylamino)phenyl)pinacolborate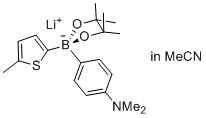  |
MeCN | N Param.: 8.02 sN Param.: 0.89 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
lithium (5-methylthiophen-2-yl)(3,5-bis(trifluoromethyl)phenyl)pinacolborate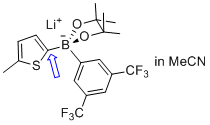  |
MeCN | N Param.: 5.53 sN Param.: 1.00 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
lithium (5-methylthiophen-2-yl)(3,5-bis(trifluoromethyl)phenyl)neopentylglycolborate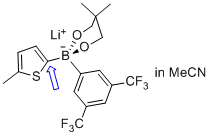  |
MeCN | N Param.: 10.13 sN Param.: 0.91 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
lithium (5-methylthiophen-2-yl)(3,5-bis(trifluoromethyl)phenyl)glycolborate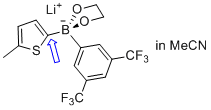  |
MeCN | N Param.: 11.23 sN Param.: 0.77 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
lithium (5-methylfuran-2-yl)(4-(trifluoromethyl)phenyl)pinacolborate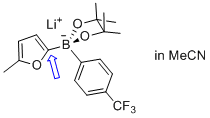  |
MeCN | N Param.: 8.13 sN Param.: 0.85 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
isobutylenyl-ethylether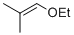  |
dichloromethane | N Param.: 4.23 sN Param.: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
Indole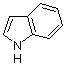  |
dichloromethane | N Param.: 5.55 sN Param.: 1.09 | J. Org. Chem. 2006, 71, 9088-9095 10.1021/jo0614339 | |
furan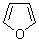  |
dichloromethane | N Param.: 1.33 sN Param.: 1.29 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | |
fluorobis(phenylsulfonyl)methanide (in DMSO)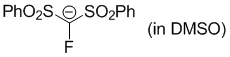  |
DMSO | N Param.: 17.46 sN Param.: 0.73 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 10.1002/anie.201605616 | |
ethyl-vinylether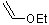  |
dichloromethane | N Param.: 3.92 sN Param.: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
ethyl diazoacetate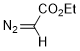  |
dichloromethane | N Param.: 4.91 sN Param.: 0.95 | Chem. Eur. J. 2003, 9, 4068-4076 10.1002/chem.200304913 | |
ethyl (E)-3-(N-morpholino)acrylate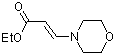  |
dichloromethane | N Param.: 8.52 sN Param.: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 10.1002/chem.200204666 | |
ethyl (E)-3-(dimethylamino)acrylate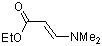  |
dichloromethane | N Param.: 9.43 sN Param.: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 10.1002/chem.200204666 | |
enolate of 3-isochromanone (in DMSO)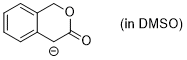  |
DMSO | N Param.: 25.39 sN Param.: 0.54 | J. Org. Chem. 2024, 89, 6915-6928 10.1021/acs.joc.4c00277 | |
enolate of 2-coumaranone (in DMSO)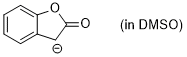  |
DMSO | N Param.: 19.60 sN Param.: 0.75 | J. Org. Chem. 2024, 89, 6915-6928 10.1021/acs.joc.4c00277 | |
diphenyldiazomethane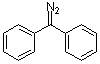  |
dichloromethane | N Param.: 5.29 sN Param.: 0.92 | Chem. Eur. J. 2003, 9, 4068-4076 10.1002/chem.200304913 | |
dimethyl diazomalonate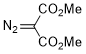  |
dichloromethane | N Param.: -1.24 sN Param.: 0.81 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
Dimethyl bis(indenyl)zirconium(IV)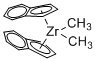  |
dichloromethane | N Param.: 6.89 sN Param.: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Dimethyl 1,1'-isopropylidenezirconocene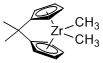  |
dichloromethane | N Param.: 5.20 sN Param.: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Dimedone iodonium ylide (in CH2Cl2)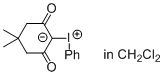  |
dichloromethane | N Param.: 6.18 sN Param.: 0.81 | J. Am. Chem. Soc. 2016, 138, 10304-10313 10.1021/jacs.6b05768 | |
diethyl diazomalonate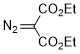  |
dichloromethane | N Param.: -0.35 sN Param.: 0.93 | Chem. Eur. J. 2003, 9, 4068-4076 10.1002/chem.200304913 | |
diazomethane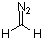  |
dichloromethane | N Param.: 10.48 sN Param.: 0.78 | Chem. Eur. J. 2003, 9, 4068-4076 10.1002/chem.200304913 | |
diazocyclopentadiene (in CH2Cl2)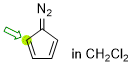  |
dichloromethane | N Param.: 4.84 sN Param.: 1.06 | Synthesis 2023, 55, 354-358 10.1055/s-0041-1737327 | |
diazoacetone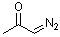  |
dichloromethane | N Param.: 3.96 sN Param.: 0.91 | Chem. Eur. J. 2003, 9, 4068-4076 10.1002/chem.200304913 | |
Dexoxy Breslow intermediate 6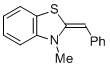  |
THF | N Param.: 15.58 sN Param.: 0.57 | Angew. Chem. Int. Ed. 2012, 51, 10408-10412 10.1002/anie.201204524 | |
Desoxy Breslow intermediate 2c'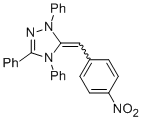  |
THF | N Param.: 12.75 sN Param.: 0.71 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 10.1002/anie.201202327 | |
Desoxy Breslow intermediate 2b'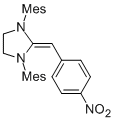  |
THF | N Param.: 11.42 sN Param.: 0.70 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 10.1002/anie.201202327 | |
Desoxy Breslow intermediate 2b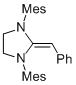  |
THF | N Param.: 13.91 sN Param.: 0.64 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 10.1002/anie.201202327 | |
Desoxy Breslow intermediate 2a'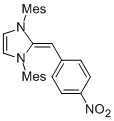  |
THF | N Param.: 14.45 sN Param.: 0.71 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 10.1002/anie.201202327 | |
Desoxy Breslow intermediate 2a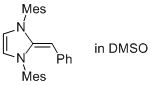  |
DMSO | N Param.: 17.41 sN Param.: 0.74 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 10.1002/anie.201202327 | |
Desoxy Breslow intermediate 2a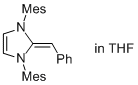  |
THF | N Param.: 17.12 sN Param.: 0.80 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 10.1002/anie.201202327 | |
Danishefskys diene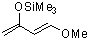  |
dichloromethane | N Param.: 8.57 sN Param.: 0.84 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
cyclopentene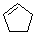  |
dichloromethane | N Param.: -1.55 sN Param.: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
cyclopentadiene  |
dichloromethane | N Param.: 2.30 sN Param.: 1.06 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
cycloocta-1,3-diene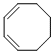  |
dichloromethane | N Param.: -0.50 sN Param.: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
cyclohexa-1,3-diene  |
dichloromethane | N Param.: 0.67 sN Param.: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
cyclohepta-1,3-diene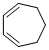  |
dichloromethane | N Param.: 0.06 sN Param.: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
cyclohepta-1,3,5-trienyl-Fe(CO)3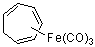  |
dichloromethane | N Param.: 3.42 sN Param.: 0.94 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
cyano(pyridin-1-ium-1-yl)methanide (in DMSO)  |
DMSO | N Param.: 25.94 sN Param.: 0.42 | J. Am. Chem. Soc. 2013, 135, 15216-15224 10.1021/ja407885h | |
cyano((diphenylmethylene)amino)methanide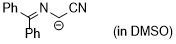  |
DMSO | N Param.: 29.50 sN Param.: 0.50 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
cyanide (in MeCN)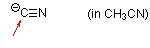  |
MeCN | N Param.: 16.27 sN Param.: 0.70 | Angew. Chem. Int. Ed. 2005, 44, 142-145 10.1002/anie.200461640 | |
Cp2Zr(Me)2 - Dimethylzirconocene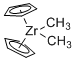  |
dichloromethane | N Param.: 4.35 sN Param.: 1.09 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Cp2Zr(CH2Ph)2 - Dibenzylzirconocene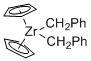  |
dichloromethane | N Param.: 5.10 sN Param.: 1.03 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Cp2Ti(CH2Ph)2 - Dibenzyltitanocene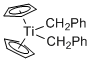  |
dichloromethane | N Param.: 4.38 sN Param.: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
chloro(phenylsulfonyl)methanide (in DMSO)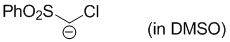  |
DMSO | N Param.: 28.27 sN Param.: 0.42 | J. Org. Chem. 2017, 82, 2011-2017 10.1021/acs.joc.6b02844 | |
chloro(phenylsulfonyl)methanide (in DMF)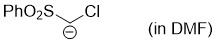  |
DMF | N Param.: 26.64 sN Param.: 0.64 | Chem. Eur. J. 2008, 14, 6108-6118 10.1002/chem.200800329 |
