C-Nucleophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
anion of 1,2,3-triphenylpropane-1,3-dione (in DMSO)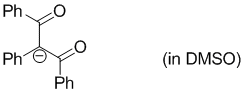  |
DMSO | N Param.: 14.99 sN Param.: 0.83 | Eur. J. Org. Chem. 2017, , 1196-1202 10.1002/ejoc.201601513 | |
barbiturate anion (in DMSO)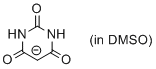  |
DMSO | N Param.: 15.59 sN Param.: 0.80 | J. Org. Chem. 2017, 82, 8476-8488 10.1021/acs.joc.7b01223 | |
1,3-dimethylbarbiturate anion (in DMSO)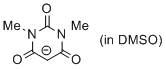  |
DMSO | N Param.: 17.46 sN Param.: 0.72 | J. Org. Chem. 2017, 82, 8476-8488 10.1021/acs.joc.7b01223 | |
2-thiobarbiturate anion (in DMSO)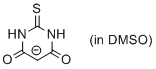  |
DMSO | N Param.: 14.24 sN Param.: 0.82 | J. Org. Chem. 2017, 82, 8476-8488 10.1021/acs.joc.7b01223 | |
1,3-diethyl-thiobarbiturate anion (in DMSO)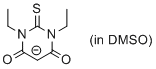  |
DMSO | N Param.: 14.90 sN Param.: 0.80 | J. Org. Chem. 2017, 82, 8476-8488 10.1021/acs.joc.7b01223 | |
4,4,5,5-tetramethyl-2-prop-2-enyl-1,3,2-dioxaborolane (in CH2Cl2)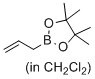  |
dichloromethane | N Param.: -0.64 sN Param.: 1.10 | J. Am. Chem. Soc. 2017, 139, 15324-15327 10.1021/jacs.7b10240 | |
lithium 2-allyl-2-(3,5-bis(trifluoromethyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-uide (in MeCN)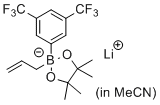  |
MeCN | N Param.: 8.71 sN Param.: 0.80 | J. Am. Chem. Soc. 2017, 139, 15324-15327 10.1021/jacs.7b10240 | |
lithium (E)-2-(3,5-bis(trifluoromethyl)phenyl)-2-(but-2-en-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-uide (in MeCN)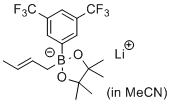  |
MeCN | N Param.: 9.49 sN Param.: 0.82 | J. Am. Chem. Soc. 2017, 139, 15324-15327 10.1021/jacs.7b10240 | |
(E)-1-(2-(4-methoxyphenyl)-1-phenylvinyl)pyrrolidine (in MeCN)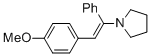  |
MeCN | N Param.: 11.99 sN Param.: 0.84 | Chem. Eur. J. 2018, 24, 5901-5910 10.1002/chem.201705962 | |
(E)-1-(1,2-diphenylvinyl)pyrrolidine (in MeCN)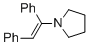  |
MeCN | N Param.: 11.66 sN Param.: 0.82 | Chem. Eur. J. 2018, 24, 5901-5910 10.1002/chem.201705962 | |
(E)-4-(2-phenyl-2-(pyrrolidin-1-yl)vinyl)benzonitrile (in MeCN)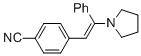  |
MeCN | N Param.: 10.63 sN Param.: 0.84 | Chem. Eur. J. 2018, 24, 5901-5910 10.1002/chem.201705962 | |
(E)-1-(2-(4-nitrophenyl)-1-phenylvinyl)pyrrolidine (in MeCN)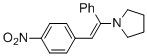  |
MeCN | N Param.: 10.42 sN Param.: 0.82 | Chem. Eur. J. 2018, 24, 5901-5910 10.1002/chem.201705962 | |
(E)-1-(1,2-diphenylvinyl)piperidine (in MeCN)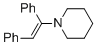  |
MeCN | N Param.: 9.94 sN Param.: 0.86 | Chem. Eur. J. 2018, 24, 5901-5910 10.1002/chem.201705962 | |
(E)-4-(1,2-diphenylvinyl)morpholine (in MeCN)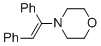  |
MeCN | N Param.: 8.78 sN Param.: 0.83 | Chem. Eur. J. 2018, 24, 5901-5910 10.1002/chem.201705962 | |
(E)-beta-(N-piperidino)styrene (in MeCN)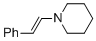  |
MeCN | N Param.: 13.84 sN Param.: 0.73 | Chem. Eur. J. 2018, 24, 5901-5910 10.1002/chem.201705962 | |
(E)-4-styrylmorpholine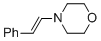  |
MeCN | N Param.: 11.66 sN Param.: 0.83 | Chem. Eur. J. 2018, 24, 5901-5910 10.1002/chem.201705962 | |
(p-nitrophenyl)diazomethane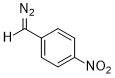  |
dichloromethane | N Param.: 7.17 sN Param.: 0.83 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
(p-cyanophenyl)diazomethane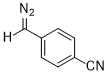  |
dichloromethane | N Param.: 7.66 sN Param.: 0.80 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
(p-bromophenyl)diazomethane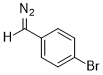  |
dichloromethane | N Param.: 8.87 sN Param.: 0.82 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
1-(trimethylsiloxy)cyclopentene (in MeCN)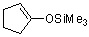  |
MeCN | N Param.: 6.43 sN Param.: 0.89 | Chem. Asian J. 2012, 7, 1401-1407 10.1002/asia.201101046 | |
1-((diphenylmethylene)amino)-2-ethoxy-2-oxoethan-1-ide (in DMSO)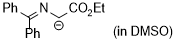  |
DMSO | N Param.: 26.95 sN Param.: 0.52 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
2-(tert-butoxy)-1-((diphenylmethylene)amino)-2-oxoethan-1-ide (in DMSO)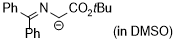  |
DMSO | N Param.: 27.77 sN Param.: 0.47 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
(Z)-1,2-diphenyl-1-(trimethylsiloxy)ethene (in MeCN)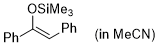  |
MeCN | N Param.: 3.00 sN Param.: 0.83 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
(Z)-1-phenyl-1-(trimethylsiloxy)propene (in MeCN)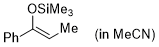  |
MeCN | N Param.: 5.18 sN Param.: 0.94 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
3-(trimethylsiloxy)-1H-indene (in MeCN)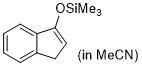  |
MeCN | N Param.: 7.32 sN Param.: 0.82 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
1-(trimethylsiloxy)-3,4-dihydronaphthalene (in MeCN)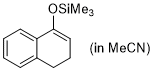  |
MeCN | N Param.: 5.06 sN Param.: 0.91 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
3-pyrrolidino-1H-indene (in MeCN)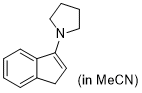  |
MeCN | N Param.: 15.27 sN Param.: 0.93 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
1-pyrrolidino-3,4-dihydronaphthalene (in MeCN)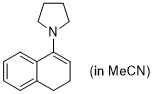  |
MeCN | N Param.: 14.09 sN Param.: 0.66 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
1-ethoxy-3-(4-nitrophenyl)-1,3-dioxopropan-2-ide (in DMSO)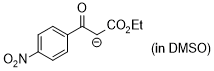  |
DMSO | N Param.: 16.26 sN Param.: 0.83 | J. Am. Chem. Soc. 2018, 140, 11474-11486 10.1021/jacs.8b07147 | |
Ph3P=CH-CHO (in MeCN)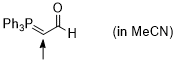  |
MeCN | N Param.: 9.09 sN Param.: 0.74 | J. Am. Chem. Soc. 2016, 138, 11272-11281 10.1021/jacs.6b06264 | |
Ph3P=CH-C(O)Me (in MeCN)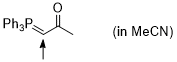  |
MeCN | N Param.: 10.27 sN Param.: 0.83 | J. Am. Chem. Soc. 2016, 138, 11272-11281 10.1021/jacs.6b06264 | |
anion of 4-(trifluoromethyl)benzyl trifluoromethyl sulfone (in MeCN)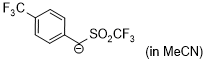  |
MeCN | N Param.: 16.15 sN Param.: 0.99 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
anion of 4-cyanobenzyl trifluoromethyl sulfone (in MeCN)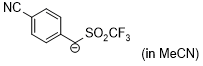  |
MeCN | N Param.: 15.62 sN Param.: 0.99 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
anion of 4-nitrobenzyl-CN (in MeCN)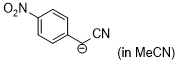  |
MeCN | N Param.: 20.10 sN Param.: 0.71 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
anion of (4-NO2-C6H4)CH2SO2Ph (in MeCN)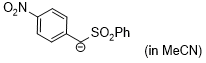  |
MeCN | N Param.: 19.90 sN Param.: 0.66 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
3-diazoindolin-2-one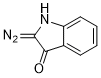  |
dichloromethane | N Param.: 3.16 sN Param.: 1.03 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazoindan-1-one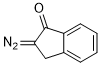  |
dichloromethane | N Param.: 5.61 sN Param.: 0.65 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazocyclohexanone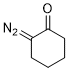  |
dichloromethane | N Param.: 3.44 sN Param.: 0.83 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-tetralone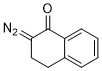  |
dichloromethane | N Param.: 3.51 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazoindandione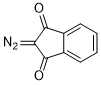  |
dichloromethane | N Param.: 0.16 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-benzosuberone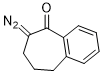  |
dichloromethane | N Param.: 2.72 sN Param.: 0.96 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazobenzothiophen-3(2H)-one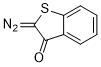  |
dichloromethane | N Param.: 0.40 sN Param.: 0.93 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
1,3-dimethyl-2-methyleneimidazolidine (in THF)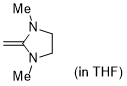  |
THF | N Param.: 18.11 sN Param.: 0.68 | J. Org. Chem. 2021, 86, 2974-2985 10.1021/acs.joc.0c02838 | |
1,3-dimethyl-2-methylenehexahydropyrimidine (in THF)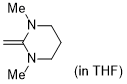  |
THF | N Param.: 18.68 sN Param.: 0.63 | J. Org. Chem. 2021, 86, 2974-2985 10.1021/acs.joc.0c02838 | |
1,3-dimesityl-2-methylene-2,3-dihydro-1H-imidazole (in THF)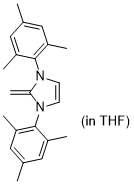  |
THF | N Param.: 17.80 sN Param.: 0.79 | J. Org. Chem. 2021, 86, 2974-2985 10.1021/acs.joc.0c02838 | |
1,3-dimethyl-2-methylene-2,3-dihydro-1H-benzo[d]imidazole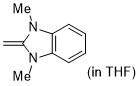  |
THF | N Param.: 19.84 sN Param.: 0.58 | J. Org. Chem. 2021, 86, 2974-2985 10.1021/acs.joc.0c02838 | |
lithium 4,4,5,5-tetramethyl-2-phenyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolan-2-uide (in MeCN)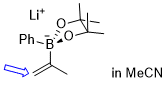  |
MeCN | N Param.: 11.00 sN Param.: 0.57 | J. Am. Chem. Soc. 2022, 144, 16118-16130 10.1021/jacs.2c06493 | |
2-((2,2,5,5-tetramethyl-1-(prop-1-en-1-yl)-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN)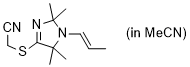  |
MeCN | N Param.: 9.25 sN Param.: 0.84 | Chem. Commun. 2023, 59, 8091-8084 10.1039/d3cc01912h | |
2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN)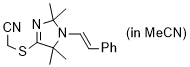  |
MeCN | N Param.: 7.74 sN Param.: 0.87 | Chem. Commun. 2023, 59, 8091-8084 10.1039/d3cc01912h | |
2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in CH2Cl2)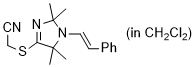  |
dichloromethane | N Param.: 7.06 sN Param.: 1.11 | Chem. Commun. 2023, 59, 8091-8084 10.1039/d3cc01912h |
