C-Nucleophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
beta-(trimethylsilyl)styrene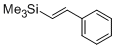  |
dichloromethane | N Param.: -0.43 sN Param.: 1.06 | Chem. Eur. J. 2014, 20, 1103-1110 10.1002/chem.201303215 | |
2-phenyl-allyltrimethylsilane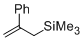  |
dichloromethane | N Param.: 5.38 sN Param.: 0.89 | Chem. Eur. J. 2014, 20, 1103-1110 10.1002/chem.201303215 | |
1-(p-tolyl)-1-(trimethylsilyl)ethene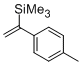  |
dichloromethane | N Param.: -0.65 sN Param.: 1.59 | Chem. Eur. J. 2014, 20, 1103-1110 10.1002/chem.201303215 | |
N-benzyl-N-(but-1-yn-1-yl)-4-methylbenzenesulfonamide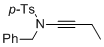  |
dichloromethane | N Param.: 5.16 sN Param.: 0.85 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 10.1002/anie.201402055 | |
N-benzyl-N-((2-methoxyphenyl)ethynyl)-4-methylbenzenesulfonamide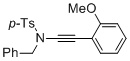  |
dichloromethane | N Param.: 4.40 sN Param.: 0.86 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 10.1002/anie.201402055 | |
4-methyl-N-(2-phenylethynyl)-N-(phenylmethyl)benzenesulfonamide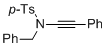  |
dichloromethane | N Param.: 3.85 sN Param.: 0.84 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 10.1002/anie.201402055 | |
1-(phenylethynyl)pyrrolidin-2-one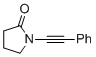  |
dichloromethane | N Param.: 3.12 sN Param.: 0.85 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 10.1002/anie.201402055 | |
(E)-1-styrylpyrrolidine (in CH2Cl2)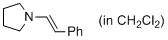  |
dichloromethane | N Param.: 12.26 sN Param.: 0.93 | J. Am. Chem. Soc. 2014, 136, 14263-14269 10.1021/ja508065e | |
(E)-1-styryl-2-tritylpyrrolidine (in CH2Cl2)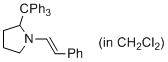  |
dichloromethane | N Param.: 10.19 sN Param.: 1.06 | J. Am. Chem. Soc. 2014, 136, 14263-14269 10.1021/ja508065e | |
(E)-1-styryl-2-(triphenylsilyl)pyrrolidine (in CH2Cl2)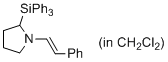  |
dichloromethane | N Param.: 11.77 sN Param.: 0.98 | J. Am. Chem. Soc. 2014, 136, 14263-14269 10.1021/ja508065e | |
(1H-inden-1-yl)trimethylstannane (in CH2Cl2)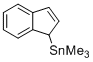  |
dichloromethane | N Param.: 6.68 sN Param.: 0.81 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 10.1002/anie.201501385 | |
(1H-inden-1-yl)trimethylsilane (in CH2Cl2)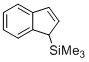  |
dichloromethane | N Param.: -0.10 sN Param.: 1.05 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 10.1002/anie.201501385 | |
Cp2Zr(Me)2 - Dimethylzirconocene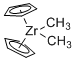  |
dichloromethane | N Param.: 4.35 sN Param.: 1.09 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Cp2Zr(CH2Ph)2 - Dibenzylzirconocene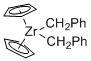  |
dichloromethane | N Param.: 5.10 sN Param.: 1.03 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Cp2Ti(CH2Ph)2 - Dibenzyltitanocene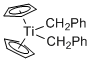  |
dichloromethane | N Param.: 4.38 sN Param.: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
(Cp*)2Zr(Me)2 - Dimethyl-bis(pentamethylcyclopentadienyl)zirconium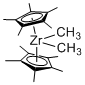  |
dichloromethane | N Param.: 5.49 sN Param.: 1.06 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Dimethyl 1,1'-isopropylidenezirconocene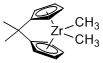  |
dichloromethane | N Param.: 5.20 sN Param.: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Dimethyl bis(indenyl)zirconium(IV)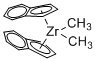  |
dichloromethane | N Param.: 6.89 sN Param.: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 10.1002/chem.201602452 | |
Meldrum's acid iodonium ylide (in CH2Cl2)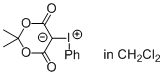  |
dichloromethane | N Param.: 4.36 sN Param.: 1.06 | J. Am. Chem. Soc. 2016, 138, 10304-10313 10.1021/jacs.6b05768 | |
Dimedone iodonium ylide (in CH2Cl2)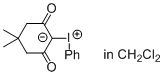  |
dichloromethane | N Param.: 6.18 sN Param.: 0.81 | J. Am. Chem. Soc. 2016, 138, 10304-10313 10.1021/jacs.6b05768 | |
N,N-Dimethylbarbituric acid iodonium ylide (in CHCl2)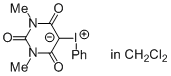  |
dichloromethane | N Param.: 7.98 sN Param.: 0.71 | J. Am. Chem. Soc. 2016, 138, 10304-10313 10.1021/jacs.6b05768 | |
6-methyl-3-(phenyl-l3-iodanylidene)dihydro-2H-pyran-2,4(3H)-dione (in CH2Cl2)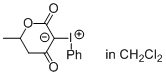  |
dichloromethane | N Param.: 4.67 sN Param.: 0.92 | J. Am. Chem. Soc. 2016, 138, 10304-10313 10.1021/jacs.6b05768 | |
4,4,5,5-tetramethyl-2-prop-2-enyl-1,3,2-dioxaborolane (in CH2Cl2)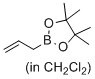  |
dichloromethane | N Param.: -0.64 sN Param.: 1.10 | J. Am. Chem. Soc. 2017, 139, 15324-15327 10.1021/jacs.7b10240 | |
(p-nitrophenyl)diazomethane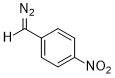  |
dichloromethane | N Param.: 7.17 sN Param.: 0.83 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
(p-cyanophenyl)diazomethane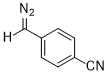  |
dichloromethane | N Param.: 7.66 sN Param.: 0.80 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
(p-bromophenyl)diazomethane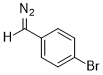  |
dichloromethane | N Param.: 8.87 sN Param.: 0.82 | J. Am. Chem. Soc. 2018, 140, 16758-16772 10.1021/jacs.8b09995 | |
3-diazoindolin-2-one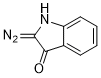  |
dichloromethane | N Param.: 3.16 sN Param.: 1.03 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazoindan-1-one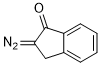  |
dichloromethane | N Param.: 5.61 sN Param.: 0.65 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazocyclohexanone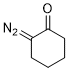  |
dichloromethane | N Param.: 3.44 sN Param.: 0.83 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-tetralone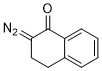  |
dichloromethane | N Param.: 3.51 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazoindandione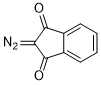  |
dichloromethane | N Param.: 0.16 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-benzosuberone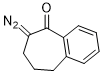  |
dichloromethane | N Param.: 2.72 sN Param.: 0.96 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazobenzothiophen-3(2H)-one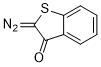  |
dichloromethane | N Param.: 0.40 sN Param.: 0.93 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in CH2Cl2)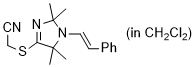  |
dichloromethane | N Param.: 7.06 sN Param.: 1.11 | Chem. Commun. 2023, 59, 8091-8084 10.1039/d3cc01912h | |
diazocyclopentadiene (in CH2Cl2)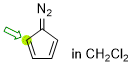  |
dichloromethane | N Param.: 4.84 sN Param.: 1.06 | Synthesis 2023, 55, 354-358 10.1055/s-0041-1737327 | |
(E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in CH2Cl2)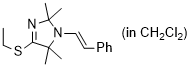  |
dichloromethane | N Param.: 8.13 sN Param.: 0.97 | Chem. Eur. J. 2024, 30, e202302764 10.1002/chem.202302764 | |
(E)-4-(benzylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in CH2Cl2)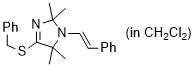  |
dichloromethane | N Param.: 7.99 sN Param.: 0.98 | Chem. Eur. J. 2024, 30, e202302764 10.1002/chem.202302764 | |
methyl diazoacetate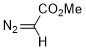  |
dichloromethane | N Param.: 4.68 sN Param.: 0.94 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
dimethyl diazomalonate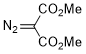  |
dichloromethane | N Param.: -1.24 sN Param.: 0.81 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
(1-azidovinyl)benzene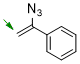  |
dichloromethane | N Param.: 4.57 sN Param.: 0.94 | Chem. Commun. 2026, , in print 10.1039/d6cc00190d | |
1-(1-azidovinyl)-4-methylbenzene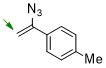  |
dichloromethane | N Param.: 5.47 sN Param.: 0.87 | Chem. Commun. 2026, , in print 10.1039/d6cc00190d | |
1-(1-azidovinyl)-4-methoxybenzene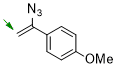  |
dichloromethane | N Param.: 6.08 sN Param.: 0.99 | Chem. Commun. 2026, , in print 10.1039/d6cc00190d | |
4-(1-azidovinyl)-1,1'-biphenyl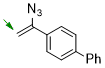  |
dichloromethane | N Param.: 5.17 sN Param.: 0.86 | Chem. Commun. 2026, , in print 10.1039/d6cc00190d | |
1-(1-azidovinyl)-4-(chloromethyl)benzene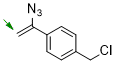  |
dichloromethane | N Param.: 4.13 sN Param.: 0.96 | Chem. Commun. 2026, , in print 10.1039/d6cc00190d | |
1-(1-azidovinyl)-4-(trifluoromethyl)benzene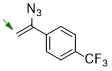  |
dichloromethane | N Param.: 3.10 sN Param.: 0.77 | Chem. Commun. 2026, , in print 10.1039/d6cc00190d | |
2-(1-azidovinyl)naphthalene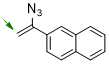  |
dichloromethane | N Param.: 5.39 sN Param.: 0.89 | Chem. Commun. 2026, , in print 10.1039/d6cc00190d | |
1-(1-azidovinyl)naphthalene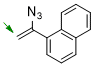  |
dichloromethane | N Param.: 3.17 sN Param.: 0.88 | Chem. Commun. 2026, , in print 10.1039/d6cc00190d | |
anion of nitroethane (in DMF)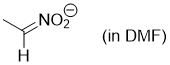  |
DMF | N Param.: 22.21 sN Param.: 0.48 | Chem. Eur. J. 2008, 14, 6108-6118 10.1002/chem.200800329 | |
chloro(phenylsulfonyl)methanide (in DMF)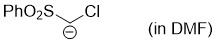  |
DMF | N Param.: 26.64 sN Param.: 0.64 | Chem. Eur. J. 2008, 14, 6108-6118 10.1002/chem.200800329 | |
anion of Meldrums acid (in DMSO)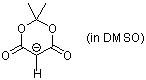  |
DMSO | N Param.: 13.91 sN Param.: 0.86 | Angew. Chem. Int. Ed. 2002, 41, 91-95 10.1002/1521-3773(20[...] |
