Electrophiles
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8
« Back Forward » Found 377 molecules, displaying page 5 of 8 Results per page
10|50|100|all
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
9-phenyl-9H-fluoren-9-ylium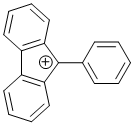  |
dichloromethane | E Param.: 2.41 | Chem. Eur. J. 2017, 23, 623-630 10.1002/chem.201603963 | |
<i>trans-beta</i>-nitrostyrene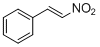  |
E Param.: -13.85 | J. Org. Chem. 2011, 76, 9370-9378 10.1021/jo201678u | ||
acrylonitrile (in DMSO)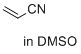  |
DMSO | E Param.: -19.05 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
ani(Br)2QM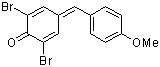  |
E Param.: -8.63 | Angew. Chem. Int. Ed. 2002, 41, 91-95 10.1002/1521-3773(20[...] | ||
ani(Ph)2QM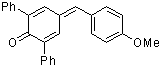  |
E Param.: -12.18 | Angew. Chem. Int. Ed. 2002, 41, 91-95 10.1002/1521-3773(20[...] | ||
ani(Ph)CH+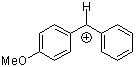  |
E Param.: 2.11 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | ||
ani(pop)CH+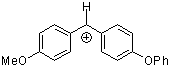  |
E Param.: 0.61 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | ||
ani(t-Bu)2QM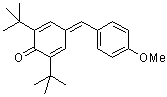  |
E Param.: -16.11 | Angew. Chem. Int. Ed. 2002, 41, 91-95 10.1002/1521-3773(20[...] | ||
ani(tol)CH+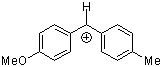  |
E Param.: 1.48 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | ||
aniBBS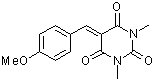  |
E Param.: -10.37 | J. Org. Chem. 2007, 72, 9170-9180 10.1021/jo071273g | ||
benzaldehyde (in DMSO)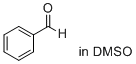  |
DMSO | E Param.: -12.90 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
benzaldehyde-boron trichloride complex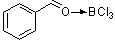  |
E Param.: 1.12 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
benzhydrylium ion Ph2CH+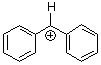  |
E Param.: 5.47 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | ||
benzylidene-Meldrum's acid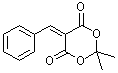  |
E Param.: -9.15 | J. Org. Chem. 2008, 73, 2738-2745 10.1021/jo702590s | ||
benzylidenemalononitrile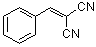  |
E Param.: -9.42 | J. Org. Chem. 2003, 68, 6880-6886 10.1021/jo0344182 | ||
bh 6f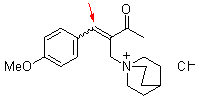  |
E Param.: -18.90 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 | ||
bh 6i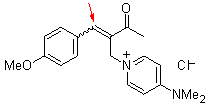  |
E Param.: -19.10 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 | ||
bh 6k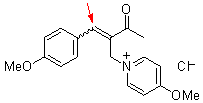  |
E Param.: -18.20 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 | ||
bh 6l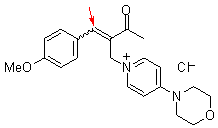  |
E Param.: -18.90 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 | ||
bh 6m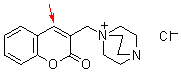  |
E Param.: -17.30 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 | ||
bh6a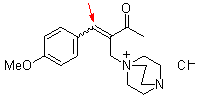  |
E Param.: -18.60 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 | ||
bh6j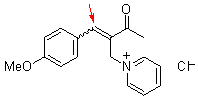  |
E Param.: -17.90 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 | ||
bis(1,2-dimethylindol-3-yl)methylium ion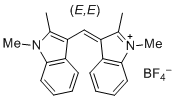  |
E Param.: -10.23 | J. Org. Chem. 2015, 80, 8643-8656 10.1021/acs.joc.5b01298 | ||
bis(1-methylindol-3-yl)methylium ion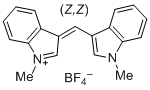  |
E Param.: -5.99 | J. Org. Chem. 2015, 80, 8643-8656 10.1021/acs.joc.5b01298 | ||
bis(5-methoxy-1-methylindol-3-yl)methylium ion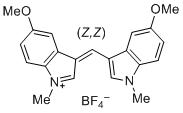  |
E Param.: -6.90 | J. Org. Chem. 2015, 80, 8643-8656 10.1021/acs.joc.5b01298 | ||
bis(julidin-9-yl)allylium ion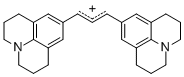  |
E Param.: -9.78 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
but-3-en-2-one (in DMSO)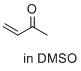  |
DMSO | E Param.: -16.76 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
butanal (in DMSO)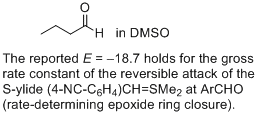  |
DMSO | J. Am. Chem. Soc. 2011, 133, 8240-8251 10.1021/ja200820m | ||
butynone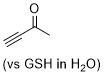  |
E Param.: -16.60 | Angew. Chem. Int. Ed. 2019, 58, 17704-17708 10.1002/anie.201909803 | ||
C6H6OMe+-Fe(CO)3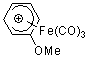  |
E Param.: -8.94 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
C6H7+-Fe(CO)3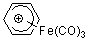  |
E Param.: -7.76 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
C7H7+-Fe(CO)3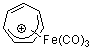  |
E Param.: -3.49 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
C7H9+-Fe(CO)3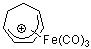  |
E Param.: -9.21 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
carbon disulfide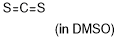  |
DMSO | E Param.: -17.70 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
cinnamaldehyde (in DMSO)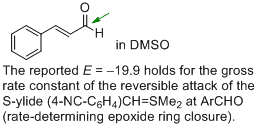  |
DMSO | J. Am. Chem. Soc. 2011, 133, 8240-8251 10.1021/ja200820m | ||
cinnamonitrile (in DMSO)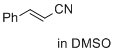  |
DMSO | E Param.: -24.60 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
cumyl cation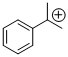  |
E Param.: 5.74 | Macromolecules 2010, 43, 1719-1723 10.1021/ma9024569 | ||
cyclobutanone (in DMSO)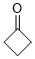  |
DMSO | E Param.: -17.50 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
cycloheptanone (in DMSO)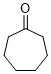  |
DMSO | E Param.: -22.10 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
cycloheptenone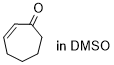  |
DMSO | E Param.: -22.00 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
cyclohexanone (in DMSO)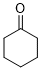  |
DMSO | E Param.: -19.90 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
cyclohexenone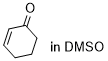  |
DMSO | E Param.: -22.10 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
cyclopentanone (in DMSO)  |
DMSO | E Param.: -21.00 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
cyclopentenone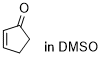  |
DMSO | E Param.: -20.60 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
di-p-anisyl-allylium ion  |
E Param.: -1.45 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
di-p-tolyl-allylium ion  |
E Param.: 1.23 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
di-tert-butyl azodicarboxylate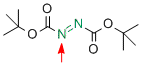  |
MeCN | E Param.: -12.23 | Chem. Eur. J. 2010, 16, 11670-11677 10.1002/chem.201001598 | |
diarylallylium ion (3,3-F2)2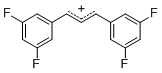  |
E Param.: 6.11 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
diarylallylium ion (3-F)2  |
E Param.: 4.15 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
diarylallylium ion (4-Br)2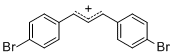  |
E Param.: 2.85 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w |
