C-Electrophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
N,N-dimethylprop-2-enamide (in DMSO)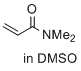  |
DMSO | E Param.: -23.54 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
ethenylsulfonylbenzene (in DMSO)  |
DMSO | E Param.: -18.36 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
acrylonitrile (in DMSO)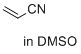  |
DMSO | E Param.: -19.05 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
but-3-en-2-one (in DMSO)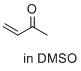  |
DMSO | E Param.: -16.76 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
1-phenylprop-2-en-1-one (in DMSO)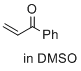  |
DMSO | E Param.: -15.25 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
ethyl 2-methylprop-2-enoate (in DMSO)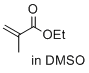  |
DMSO | E Param.: -22.77 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
ethyl (E)-but-2-enoate (in DMSO)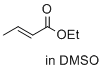  |
DMSO | E Param.: -23.59 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
ethyl cinnamate (in DMSO)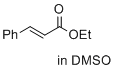  |
DMSO | E Param.: -24.52 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
(E)-1-methyl-4-(styrylsulfonyl)benzene (in DMSO)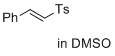  |
DMSO | E Param.: -24.69 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
cyclobutanone (in DMSO)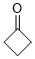  |
DMSO | E Param.: -17.50 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
cyclopentanone (in DMSO)  |
DMSO | E Param.: -21.00 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
cyclohexanone (in DMSO)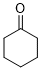  |
DMSO | E Param.: -19.90 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
cycloheptanone (in DMSO)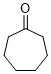  |
DMSO | E Param.: -22.10 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
1-methylpiperidin-4-one (in DMSO)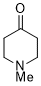  |
DMSO | E Param.: -18.40 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
oxan-4-one (in DMSO)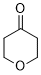  |
DMSO | E Param.: -17.90 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
thian-4-one (in DMSO)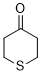  |
DMSO | E Param.: -16.90 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
1,4-dioxaspiro[4.5]decan-8-one (in DMSO)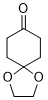  |
DMSO | E Param.: -18.20 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
pentan-2-one (in DMSO)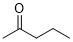  |
DMSO | E Param.: -22.30 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
1-methylsulfanylpropan-2-one (in DMSO)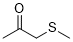  |
DMSO | E Param.: -15.60 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
phenyl isocyanate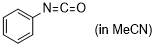  |
MeCN | E Param.: -15.38 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
p-tosyl isocyanate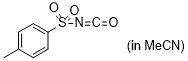  |
MeCN | E Param.: -7.69 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
phenyl isothiocyanate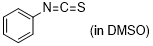  |
DMSO | E Param.: -18.15 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
p-nitrophenyl isothiocyanate  |
DMSO | E Param.: -15.89 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
diphenylcarbodiimide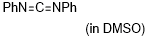  |
DMSO | E Param.: -20.14 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
carbon disulfide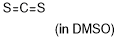  |
DMSO | E Param.: -17.70 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
benzylidenemalononitrile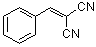  |
E Param.: -9.42 | J. Org. Chem. 2003, 68, 6880-6886 10.1021/jo0344182 | ||
p-(methoxy)benzylidenemalononitrile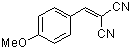  |
E Param.: -10.80 | J. Org. Chem. 2003, 68, 6880-6886 10.1021/jo0344182 | ||
p-(dimethylamino)benzylidenemalononitrile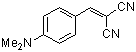  |
E Param.: -13.30 | J. Org. Chem. 2003, 68, 6880-6886 10.1021/jo0344182 | ||
4,6-dinitrobenzofuroxan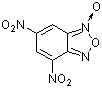  |
E Param.: -5.06 | J. Org. Chem. 2006, 71, 9088-9095 10.1021/jo0614339 | ||
1,3,5-trinitrobenzene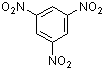  |
E Param.: -13.19 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
6-nitro-tetrazolo[1,5a]pyridine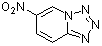  |
E Param.: -9.05 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
4-nitro-6-(trifluoromethylsulfonyl)benzofuroxan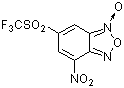  |
E Param.: -4.91 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
4,6-dinitrotetrazolopyridine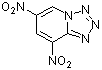  |
E Param.: -4.67 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
4,6-dinitrobenzofurazan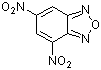  |
E Param.: -5.46 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
2,4-dinitrothiophene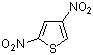  |
E Param.: -12.33 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
6-cyano-4-nitrobenzofuroxan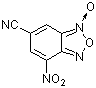  |
E Param.: -7.01 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
4-cyano-6-nitro-benzofuroxane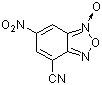  |
E Param.: -6.41 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
4-aza-6-nitrobenzofuroxan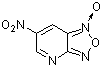  |
E Param.: -5.86 | J. Org. Chem. 2005, 70, 6242-6253 10.1021/jo0505526 | ||
aniBBS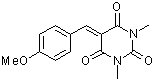  |
E Param.: -10.37 | J. Org. Chem. 2007, 72, 9170-9180 10.1021/jo071273g | ||
dmaBBS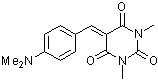  |
E Param.: -12.76 | J. Org. Chem. 2007, 72, 9170-9180 10.1021/jo071273g | ||
julBBS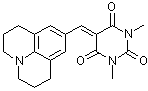  |
E Param.: -13.84 | J. Org. Chem. 2007, 72, 9170-9180 10.1021/jo071273g | ||
dma(S)BBS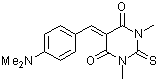  |
E Param.: -10.73 | J. Org. Chem. 2007, 72, 9170-9180 10.1021/jo071273g | ||
jul(S)BBS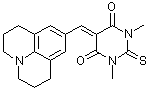  |
E Param.: -11.89 | J. Org. Chem. 2007, 72, 9170-9180 10.1021/jo071273g | ||
benzylidene-Meldrum's acid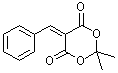  |
E Param.: -9.15 | J. Org. Chem. 2008, 73, 2738-2745 10.1021/jo702590s | ||
p-(methoxy)benzylidene Meldrum's acid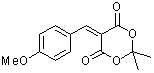  |
E Param.: -10.28 | J. Org. Chem. 2008, 73, 2738-2745 10.1021/jo702590s | ||
p-(dimethylamino)benzylidene Meldrum's acid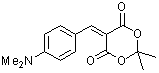  |
E Param.: -12.76 | J. Org. Chem. 2008, 73, 2738-2745 10.1021/jo702590s | ||
jul Meldrum's acid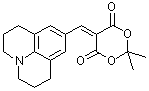  |
E Param.: -13.97 | J. Org. Chem. 2008, 73, 2738-2745 10.1021/jo702590s | ||
diarylallylium ion (3,3-F2)2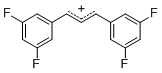  |
E Param.: 6.11 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
diarylallylium ion (3-F)2  |
E Param.: 4.15 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
diarylallylium ion (4-Br)2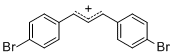  |
E Param.: 2.85 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w |
