N-Nucleophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
1-methyl-imidazole (in MeCN)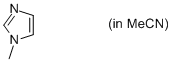  |
MeCN | N Param.: 11.90 sN Param.: 0.73 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
1-methyl-imidazole (in water)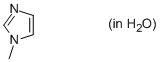  |
water | N Param.: 9.91 sN Param.: 0.55 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
1-phenyl-imidazole (in MeCN)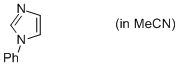  |
MeCN | N Param.: 11.31 sN Param.: 0.67 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
1-phenyl-N-(phenylmethyl)methanimine (in CH2Cl2)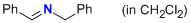  |
dichloromethane | N Param.: 7.90 sN Param.: 0.76 | Z. Naturforsch. B 2013, 68b, 693-699 10.5560/ZNB.2013-3085 | |
2,2,2-trifluoroethylamine (in 91M9AN)  |
MeOH-MeCN mix | N Param.: 10.20 sN Param.: 0.92 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 10.1002/anie.200600542 | |
2,2,2-trifluoroethylamine (in DMSO)  |
DMSO | N Param.: 12.15 sN Param.: 0.65 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | |
2,2,2-trifluoroethylamine (in MeCN)  |
MeCN | N Param.: 10.13 sN Param.: 0.75 | Eur. J. Org. Chem. 2009, , 6379-6385 10.1002/ejoc.200900925 | |
2,2-dimethylpyrrolidine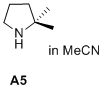  |
MeCN | N Param.: 13.96 sN Param.: 0.76 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
2,3,4,6,7,8-hexahydro-1H-pyrimido[1,2-a]pyrimidine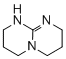  |
dichloromethane | N Param.: 16.16 sN Param.: 0.75 | ChemCatChem 2012, 4, 993-999 10.1002/cctc.201200143 | |
2,3,4,6,7,8-hexahydropyrimido[2,1-b][1,3]thiazine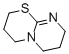  |
dichloromethane | N Param.: 14.10 sN Param.: 0.82 | J. Org. Chem. 2011, 76, 5104-5112 10.1021/jo200803x | |
2,3,5,6-tetrahydro-1H-imidazo[1,2-a]imidazole (TBO)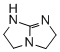  |
dichloromethane | N Param.: 14.44 sN Param.: 0.79 | ChemCatChem 2012, 4, 993-999 10.1002/cctc.201200143 | |
2,3,5,6-tetrahydroimidazo[2,1-b]thiazole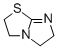  |
dichloromethane | N Param.: 12.98 sN Param.: 0.81 | J. Org. Chem. 2011, 76, 5104-5112 10.1021/jo200803x | |
2,3-dihydrobenzo[d]imidazo[2,1-b]thiazole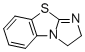  |
dichloromethane | N Param.: 13.42 sN Param.: 0.73 | J. Org. Chem. 2011, 76, 5104-5112 10.1021/jo200803x | |
2,4,6-trimethylpyridine (in CH2Cl2)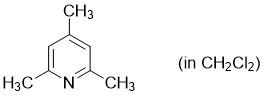  |
dichloromethane | N Param.: 9.34 sN Param.: 0.71 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,4,6-trimethylpyridine (in MeCN)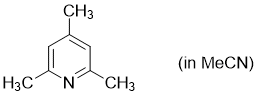  |
MeCN | N Param.: 9.39 sN Param.: 0.60 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,4-dimethyl-imidazole (in MeCN)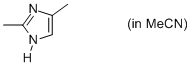  |
MeCN | N Param.: 11.51 sN Param.: 0.84 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
2,4-dimethyl-imidazole anion (in DMSO)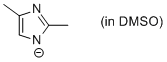  |
DMSO | N Param.: 20.69 sN Param.: 0.60 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
2,5-dimethyl-benzimidazole (in DMSO)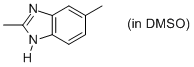  |
DMSO | N Param.: 10.21 sN Param.: 0.85 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
2,6-dimethylpyridine (in CH2Cl2)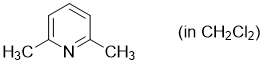  |
dichloromethane | N Param.: 9.87 sN Param.: 0.68 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,6-dimethylpyridine (in MeCN)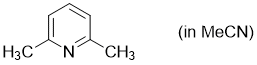  |
MeCN | N Param.: 9.11 sN Param.: 0.69 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2-(naphthylmethyl)quinuclidine (in MeCN)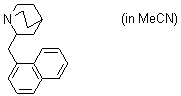  |
MeCN | N Param.: 15.66 sN Param.: 0.62 | J. Org. Chem. 2009, 74, 7157-7164 10.1021/jo901670w | |
2-(trifluoromethyl)pyrrolidine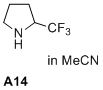  |
MeCN | N Param.: 11.34 sN Param.: 0.73 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
2-(triphenylsilyl)pyrrolidine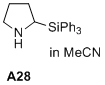  |
MeCN | N Param.: 14.00 sN Param.: 0.84 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
2-Ac super-dmap (in MeCN)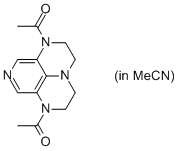  |
MeCN | N Param.: 15.39 sN Param.: 0.60 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-aminobutan-1-ol (in DMSO)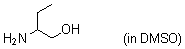  |
DMSO | N Param.: 14.39 sN Param.: 0.67 | J. Am. Chem. Soc. 2009, 131, 11392-11401 10.1021/ja903207b | |
2-benzyl-1,1,3,3-tetramethylguanidine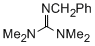  |
dichloromethane | N Param.: 14.36 sN Param.: 0.79 | ChemCatChem 2012, 4, 993-999 10.1002/cctc.201200143 | |
2-benzylpyrrolidine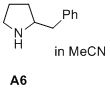  |
MeCN | N Param.: 17.43 sN Param.: 0.66 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
2-Bn super-dmap (in MeCN)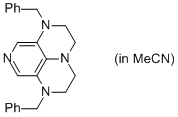  |
MeCN | N Param.: 17.69 sN Param.: 0.57 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-Bz super-dmap (in MeCN)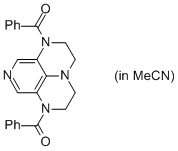  |
MeCN | N Param.: 14.19 sN Param.: 0.67 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-Et super-dmap (in MeCN)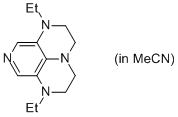  |
MeCN | N Param.: 16.81 sN Param.: 0.60 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-formyl-imidazole anion (in DMSO)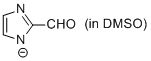  |
DMSO | N Param.: 16.06 sN Param.: 0.68 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
2-formyl-imidazole anion (in water)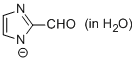  |
water | N Param.: 11.07 sN Param.: 0.50 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
2-Me super-dmap (in MeCN)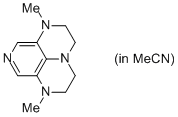  |
MeCN | N Param.: 16.65 sN Param.: 0.58 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-methyl-1,4,5,6-tetrahydropyrimidine (in CH2Cl2)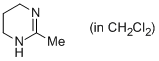  |
dichloromethane | N Param.: 15.21 sN Param.: 0.69 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-methyl-1,4,5,6-tetrahydropyrimidine (in DMSO)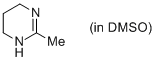  |
DMSO | N Param.: 14.58 sN Param.: 0.79 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-methyl-1,4,5,6-tetrahydropyrimidine (in MeCN)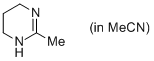  |
MeCN | N Param.: 14.43 sN Param.: 0.79 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-methyl-4,5-dihydro-1,3-oxazole (in CH2Cl2)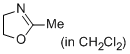  |
dichloromethane | N Param.: 9.81 sN Param.: 0.77 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-methyl-4,5-dihydro-1H-imidazole (in CH2Cl2)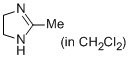  |
dichloromethane | N Param.: 12.92 sN Param.: 0.77 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-methyl-4,5-dihydro-3H-pyrrole (in CH2Cl2)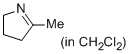  |
dichloromethane | N Param.: 13.12 sN Param.: 0.69 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-methyl-4,5-dihydrothiazole (in CH2Cl2)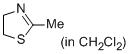  |
dichloromethane | N Param.: 10.20 sN Param.: 0.71 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-methyl-benzimidazole (in DMSO)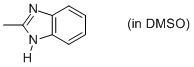  |
DMSO | N Param.: 10.02 sN Param.: 0.85 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
2-methyl-imidazole (in MeCN)  |
MeCN | N Param.: 11.74 sN Param.: 0.76 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
2-methyl-imidazole (in water)  |
water | N Param.: 9.45 sN Param.: 0.54 | Org. Biomol. Chem. 2010, 8, 1929-1935 10.1039/c000965b | |
2-methyl-imidazole anion (in DMSO)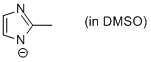  |
DMSO | N Param.: 21.03 sN Param.: 0.50 | Chem. Eur. J. 2012, 18, 127-137 10.1002/chem.201102411 | |
2-methylpyridine (in CH2Cl2)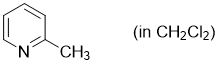  |
dichloromethane | N Param.: 10.52 sN Param.: 0.78 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2-methylpyridine (in MeCN)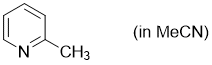  |
MeCN | N Param.: 10.98 sN Param.: 0.66 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2-methylpyrrolidine (in MeCN)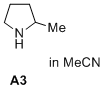  |
MeCN | N Param.: 16.78 sN Param.: 0.71 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
2-phenyl-1,4,5,6-tetrahydropyrimidine (in CH2Cl2)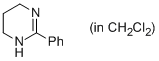  |
dichloromethane | N Param.: 14.62 sN Param.: 0.72 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-phenyl-4,5-dihydro-1H-imidazole (in CH2Cl2)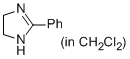  |
dichloromethane | N Param.: 12.31 sN Param.: 0.77 | Eur. J. Org. Chem. 2013, , 3369-3377 10.1002/ejoc.201300213 | |
2-phenylethylamine (in water)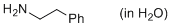  |
water | N Param.: 13.40 sN Param.: 0.57 | ChemPlusChem 2015, 80, 1673-1679 10.1002/cplu.201500246 |
