Carbocations
1 | 2 | 3
« Back Forward » Found 134 molecules, displaying page 1 of 3 Results per page
10|50|100|all
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
Vilsmeier ion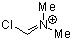  |
E Param.: -5.77 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
tropylium ion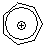  |
E Param.: -3.72 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
tritylium ion (Ph3C+)  |
E Param.: 0.51 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | ||
tol(Ph)CH+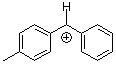  |
E Param.: 4.43 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | ||
pyrN+=CH-CH-Ph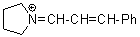  |
E Param.: -9.80 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
pTolCH=N+CH2 (in MeCN)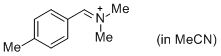  |
MeCN | E Param.: -9.64 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
pop(tol)CH+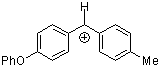  |
E Param.: 2.16 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
pop(Ph)CH+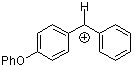  |
E Param.: 2.90 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | ||
pipN+=CH-CH-Ph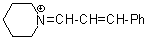  |
E Param.: -10.30 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
PhCH=N+Me2 (in MeCN)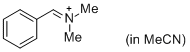  |
MeCN | E Param.: -9.27 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
PhCH=N+(CH2)5 (in MeCN)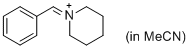  |
MeCN | E Param.: -9.60 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
PhCH=N+(CH2)4 (in MeCN)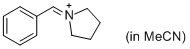  |
MeCN | E Param.: -9.35 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
Ph2C=C=N(+)Me2  |
E Param.: -1.90 | Synthesis 2025, 57, 3251-3262 10.1055/a-2649-1999 | ||
pfp(Ph)CH+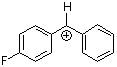  |
E Param.: 5.20 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | ||
p-CF3-C6H4-CH=N+Me2 (in MeCN)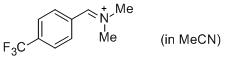  |
MeCN | E Param.: -8.34 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
p-AniCH=N+Me2 (in MeCN)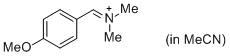  |
MeCN | E Param.: -10.69 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
N-methylacridinium ion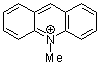  |
E Param.: -7.15 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
N-ethylidenecarbazolium ion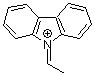  |
E Param.: 2.41 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
mor iminium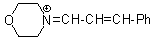  |
E Param.: -8.60 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
methyl(phenyl)methyleneammonium ion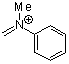  |
E Param.: -5.17 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
methoxy-phenylmethylium ion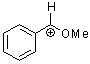  |
E Param.: 2.97 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
methoxy-(4-methylphenyl)methylium ion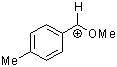  |
E Param.: 1.90 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
methoxy-(4-methoxyphenyl)methylium ion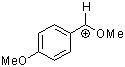  |
E Param.: 0.14 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
Me2N+=CH-CH-Ph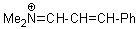  |
E Param.: -9.20 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
Me2C=C=N(+)Me2  |
E Param.: -2.80 | Synthesis 2025, 57, 3251-3262 10.1055/a-2649-1999 | ||
MacMillan-iminium ion (spiro)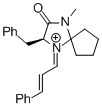  |
MeCN | E Param.: -7.67 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 10.1002/anie.201103683 | |
MacMillan-iminium ion (2nd generation)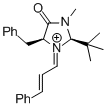  |
MeCN | E Param.: -5.52 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 10.1002/anie.201103683 | |
MacMillan-iminium ion (1st generation)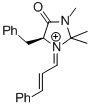  |
MeCN | E Param.: -7.37 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 10.1002/anie.201103683 | |
Jorgensen/Hayashi-iminium ion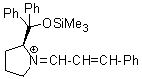  |
E Param.: -8.20 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
indolecarboxylate derived iminium ion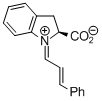  |
dichloromethane | E Param.: -9.50 | Angew. Chem. Int. Ed. 2009, 48, 5034-5037 10.1002/anie.200900933 | |
flavylium ion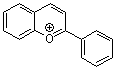  |
E Param.: -3.45 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
diphenylallylium ion  |
E Param.: 2.70 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
dimethylmethyleneammonium ion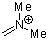  |
E Param.: -6.69 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
dibenzo[a,d]tropylium ion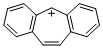  |
dichloromethane | E Param.: -0.63 | Liebigs Ann. 1995, , 2005-2009 10.1002/jlac.1995199[...] | |
diarylallylium ion (4-NMe2)2  |
E Param.: -7.50 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
diarylallylium ion (4-Cl)2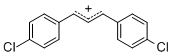  |
E Param.: 2.69 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
diarylallylium ion (4-Br)2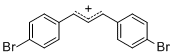  |
E Param.: 2.85 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
diarylallylium ion (3-F)2  |
E Param.: 4.15 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
diarylallylium ion (3,3-F2)2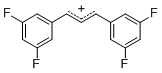  |
E Param.: 6.11 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
di-p-tolyl-allylium ion  |
E Param.: 1.23 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
di-p-anisyl-allylium ion  |
E Param.: -1.45 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
cumyl cation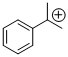  |
E Param.: 5.74 | Macromolecules 2010, 43, 1719-1723 10.1021/ma9024569 | ||
bis(julidin-9-yl)allylium ion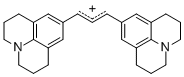  |
E Param.: -9.78 | J. Org. Chem. 2011, 76, 9391-9408 10.1021/jo201668w | ||
bis(5-methoxy-1-methylindol-3-yl)methylium ion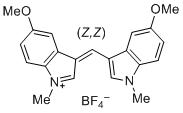  |
E Param.: -6.90 | J. Org. Chem. 2015, 80, 8643-8656 10.1021/acs.joc.5b01298 | ||
bis(1-methylindol-3-yl)methylium ion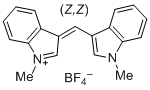  |
E Param.: -5.99 | J. Org. Chem. 2015, 80, 8643-8656 10.1021/acs.joc.5b01298 | ||
bis(1,2-dimethylindol-3-yl)methylium ion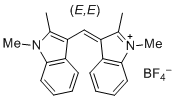  |
E Param.: -10.23 | J. Org. Chem. 2015, 80, 8643-8656 10.1021/acs.joc.5b01298 | ||
benzhydrylium ion Ph2CH+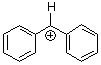  |
E Param.: 5.47 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | ||
ani(tol)CH+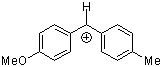  |
E Param.: 1.48 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | ||
ani(pop)CH+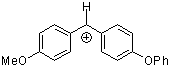  |
E Param.: 0.61 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | ||
ani(Ph)CH+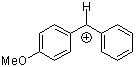  |
E Param.: 2.11 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y |
