Electrophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
carbon disulfide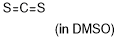  |
DMSO | E Param.: -17.70 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
dicarbonyl(cyclopentadienyl)propene-iron(II)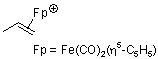  |
E Param.: -11.20 | Helv. Chim. Acta 2005, 88, 1754-1768 10.1002/hlca.200590137 | ||
2,6-diphenyl-4-benzylidenecyclohexa-2,5-dienone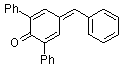  |
E Param.: -11.87 | Eur. J. Org. Chem. 2009, , 3203-3211 10.1002/ejoc.200900299 | ||
2,6-dimethoxy-4-(4-methoxybenzylidene)cyclohexa-2,5-dienone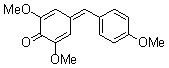  |
E Param.: -16.38 | Eur. J. Org. Chem. 2009, , 3203-3211 10.1002/ejoc.200900299 | ||
2,6-dimethoxy-4-(4-(dimethylamino)benzylidene)cyclohexa-2,5-dienone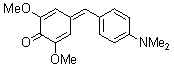  |
E Param.: -17.18 | Eur. J. Org. Chem. 2009, , 3203-3211 10.1002/ejoc.200900299 | ||
2,6-dimethyl-4-(4-(dimethylamino)benzylidene)cyclohexa-2,5-dienone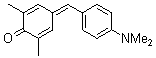  |
E Param.: -16.36 | Eur. J. Org. Chem. 2009, , 3203-3211 10.1002/ejoc.200900299 | ||
2,6-di-tert.butyl-4-(3-fluorobenzylidene)cyclohexa-2,5-dienone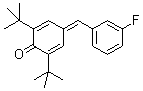  |
E Param.: -15.03 | Eur. J. Org. Chem. 2009, , 3203-3211 10.1002/ejoc.200900299 | ||
2,6-di-tert.butyl-4-(3,5-difluorobenzylidene)cyclohexa-2,5-dienone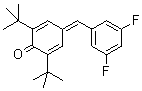  |
E Param.: -14.50 | Eur. J. Org. Chem. 2009, , 3203-3211 10.1002/ejoc.200900299 | ||
2,6-di-tert.butyl-4-(4-nitrobenzylidene)cyclohexa-2,5-dienone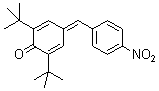  |
E Param.: -14.36 | Eur. J. Org. Chem. 2009, , 3203-3211 10.1002/ejoc.200900299 | ||
(4-MeO)-tritylium ion  |
E Param.: -1.59 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(4-F)-tritylium ion  |
E Param.: 0.35 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(4-F)2-tritylium ion  |
E Param.: 0.17 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(4-F)3-tritylium ion  |
E Param.: 0.05 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(3-F)-tritylium ion  |
E Param.: 1.01 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(3-F)2-tritylium ion  |
E Param.: 1.54 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(3-F)3-tritylium ion  |
E Param.: 2.07 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(3-F)4-tritylium ion  |
E Param.: 2.54 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
maleic anhydride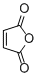  |
DMSO | E Param.: -11.31 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
N-methyl maleimide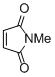  |
DMSO | E Param.: -14.07 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
fumaronitrile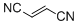  |
DMSO | E Param.: -15.71 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
diethyl fumarate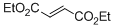  |
DMSO | E Param.: -17.79 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
diethyl maleate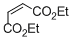  |
DMSO | E Param.: -19.49 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
(E)-2-(tert-butyl)-3-(4-methylbenzylidene)-3H-indol-1-ium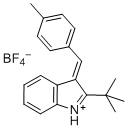  |
dichloromethane | E Param.: -5.06 | Eur. J. Org. Chem. 2016, , 4050-4058 10.1002/ejoc.201600572 | |
2,6-di-tert-butyl-4-(2,2,2-trifluoroethylidene)cyclohexa-2,5-dien-1-one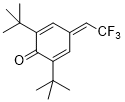  |
DMSO | E Param.: -11.68 | Eur. J. Org. Chem. 2020, , 3812-3817 10.1002/ejoc.202000295 | |
Umemoto I (triflate)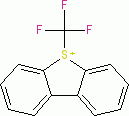  |
E Param.: -13.08 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
Umemoto I (tetrafluoroborate)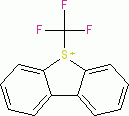  |
E Param.: -13.39 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
Umemoto II (triflate)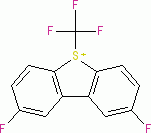  |
E Param.: -12.80 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
3-methylcyclopentenone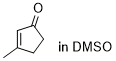  |
DMSO | E Param.: -28.90 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
2-methylcyclopentenone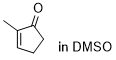  |
DMSO | E Param.: -22.10 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
cyclopentenone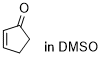  |
DMSO | E Param.: -20.60 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
furan-2(5H)-one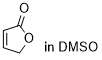  |
DMSO | E Param.: -20.70 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
2-methylcyclohexenone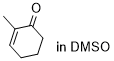  |
DMSO | E Param.: -27.50 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
cyclohexenone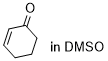  |
DMSO | E Param.: -22.10 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
dihydro-2H-pyran-2-one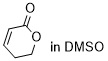  |
DMSO | E Param.: -21.80 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
3-methylenetetrahydropyran-2-one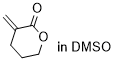  |
DMSO | E Param.: -19.50 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
3-methylenedihydrofuranone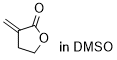  |
DMSO | E Param.: -19.40 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
3-methylcyclohexenone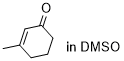  |
DMSO | E Param.: -29.60 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
cycloheptenone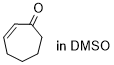  |
DMSO | E Param.: -22.00 | Chem. Sci. 2021, 12, 4850-4865 10.1039/D0SC06628A | |
4-nitrobenzodifuroxan (NBDF)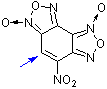  |
E Param.: -6.15 | Chem. Eur. J. 2007, 13, 8317-8324 10.1002/chem.200700676 | ||
diethyl benzylidene malonate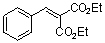  |
E Param.: -20.55 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
diethyl 4-nitrobenzylidene malonate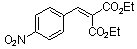  |
E Param.: -17.67 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
diethyl 4-cyano-benzylidene malonate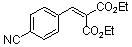  |
E Param.: -18.06 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
diethyl 3-chloro-benzylidene malonate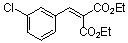  |
E Param.: -18.98 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
diethyl 4-methyl-benzylidene malonate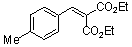  |
E Param.: -21.11 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
diethyl 4-methoxy-benzylidene malonate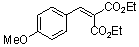  |
E Param.: -21.47 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
diethyl 4-(dimethylamino)benzylidene malonate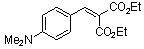  |
E Param.: -23.10 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
diethyl 2-((1-methyl-1,2,3,4-tetrahydroquinolin-6-yl)methylene)malonate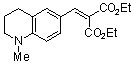  |
E Param.: -23.40 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
diethyl 2-((julolidine-9-yl)methylene)malonate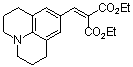  |
E Param.: -23.80 | Chem. Eur. J. 2008, 14, 9675-9682 10.1002/chem.200801277 | ||
bh6a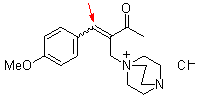  |
E Param.: -18.60 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 | ||
bh 6f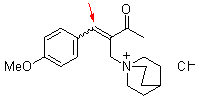  |
E Param.: -18.90 | Chem. Eur. J. 2010, 16, 1365-1371 10.1002/chem.200902487 |
