Nucleophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
1-(4-(tert-butyl)phenyl)-2-methylene-4,6-diphenyl-1,2-dihydropyridine (in DMSO)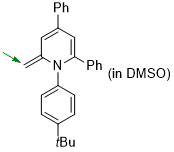  |
DMSO | N Param.: 19.36 sN Param.: 0.68 | Eur. J. Org. Chem. 2024, 27, e202400373 10.1002/ejoc.202400373 | |
enolate of 3-isochromanone (in DMSO)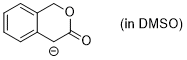  |
DMSO | N Param.: 25.39 sN Param.: 0.54 | J. Org. Chem. 2024, 89, 6915-6928 10.1021/acs.joc.4c00277 | |
enolate of 2-coumaranone (in DMSO)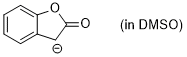  |
DMSO | N Param.: 19.60 sN Param.: 0.75 | J. Org. Chem. 2024, 89, 6915-6928 10.1021/acs.joc.4c00277 | |
cyanoborate ion (in water)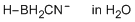  |
water | N Param.: 9.99 sN Param.: 0.54 | Org. Biomol. Chem. 2023, 21, 85-88 10.1039/D2OB02041F | |
3-diazoindolin-2-one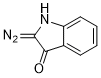  |
dichloromethane | N Param.: 3.16 sN Param.: 1.03 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
NADPH (in water)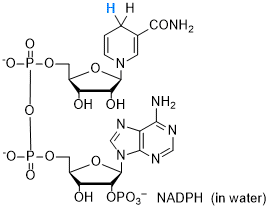  |
water | N Param.: 10.68 sN Param.: 0.54 | Org. Biomol. Chem. 2023, 21, 85-88 10.1039/D2OB02041F | |
2-diazoindan-1-one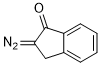  |
dichloromethane | N Param.: 5.61 sN Param.: 0.65 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazocyclohexanone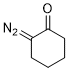  |
dichloromethane | N Param.: 3.44 sN Param.: 0.83 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-tetralone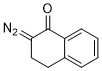  |
dichloromethane | N Param.: 3.51 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazoindandione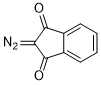  |
dichloromethane | N Param.: 0.16 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-benzosuberone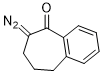  |
dichloromethane | N Param.: 2.72 sN Param.: 0.96 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazobenzothiophen-3(2H)-one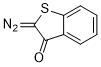  |
dichloromethane | N Param.: 0.40 sN Param.: 0.93 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
NADH (in water)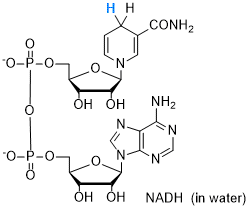  |
water | N Param.: 10.37 sN Param.: 0.54 | Org. Biomol. Chem. 2023, 21, 85-88 10.1039/D2OB02041F | |
2-((2,2,5,5-tetramethyl-1-(prop-1-en-1-yl)-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN)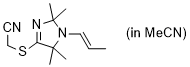  |
MeCN | N Param.: 9.25 sN Param.: 0.84 | Chem. Commun. 2023, 59, 8091-8084 10.1039/d3cc01912h | |
2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN)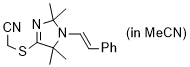  |
MeCN | N Param.: 7.74 sN Param.: 0.87 | Chem. Commun. 2023, 59, 8091-8084 10.1039/d3cc01912h | |
2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in CH2Cl2)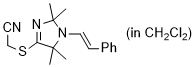  |
dichloromethane | N Param.: 7.06 sN Param.: 1.11 | Chem. Commun. 2023, 59, 8091-8084 10.1039/d3cc01912h | |
1,3-Bis(2,6-diisopropylphenyl)-5-methylene-4-(2,4,6-triisopropylphenyl)-4,5-dihydro-1H-1,2,3-triazol-3-ium-4-ide (in THF)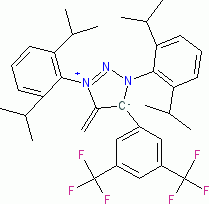  |
THF | N Param.: 21.36 sN Param.: 0.42 | Angew. Chem. Int. Ed. 2023, 62, e202309790 10.1002/anie.202309790 | |
1,3-Bis(2,6-diisopropylphenyl)-4-methylene-5-(4-(trifluoromethyl)phenyl)-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF)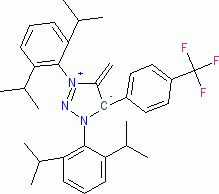  |
THF | N Param.: 22.30 sN Param.: 0.49 | Angew. Chem. Int. Ed. 2023, 62, e202309790 10.1002/anie.202309790 | |
1,3-Bis(2,6-diisopropylphenyl)-5-(4-methoxyphenyl)-4-methylene-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF)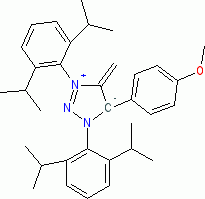  |
THF | N Param.: 23.55 sN Param.: 0.56 | Angew. Chem. Int. Ed. 2023, 62, e202309790 10.1002/anie.202309790 | |
1,3-Bis(2,6-diisopropylphenyl)-5-methylene-4-(2,4,6-triisopropylphenyl)-4,5-dihydro-1H-1,2,3-triazol-3-ium-4-ide (in THF)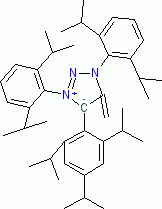  |
THF | N Param.: 20.78 sN Param.: 0.61 | Angew. Chem. Int. Ed. 2023, 62, e202309790 10.1002/anie.202309790 | |
1,3-Diisopropyl-4-methylene-5-phenyl-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF)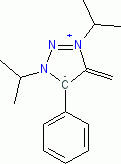  |
THF | N Param.: 30.25 sN Param.: 0.54 | Angew. Chem. Int. Ed. 2023, 62, e202309790 10.1002/anie.202309790 | |
5-(tert-Butyl)-1,3-diisopropyl-4-methylene-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF)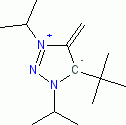  |
THF | N Param.: 31.92 sN Param.: 0.52 | Angew. Chem. Int. Ed. 2023, 62, e202309790 10.1002/anie.202309790 | |
1,3-bis(2,6-diisopropylphenyl)-4-methylene-5-phenyl-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF)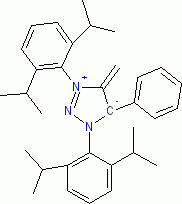  |
THF | N Param.: 22.80 sN Param.: 0.60 | Angew. Chem. Int. Ed. 2023, 62, e202309790 10.1002/anie.202309790 | |
1,3-bis(2,6-diisopropylphenyl)-5-methyl-4-methylene-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF)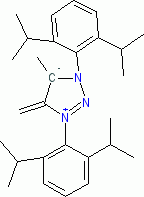  |
THF | N Param.: 24.86 sN Param.: 0.52 | Angew. Chem. Int. Ed. 2023, 62, e202309790 10.1002/anie.202309790 | |
diazocyclopentadiene (in CH2Cl2)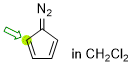  |
dichloromethane | N Param.: 4.84 sN Param.: 1.06 | Synthesis 2023, 55, 354-358 10.1055/s-0041-1737327 | |
methyl diazoacetate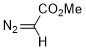  |
dichloromethane | N Param.: 4.68 sN Param.: 0.94 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
dimethyl diazomalonate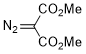  |
dichloromethane | N Param.: -1.24 sN Param.: 0.81 | J. Am. Chem. Soc. 2023, 145, 7416-7434 10.1021/jacs.2c13872 | |
lithium 4,4,5,5-tetramethyl-2-phenyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolan-2-uide (in MeCN)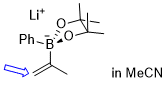  |
MeCN | N Param.: 11.00 sN Param.: 0.57 | J. Am. Chem. Soc. 2022, 144, 16118-16130 10.1021/jacs.2c06493 | |
dimethylsulfide (in CH2Cl2)  |
dichloromethane | N Param.: 12.32 sN Param.: 0.72 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
dimethylsulfide (in MeCN)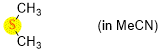  |
MeCN | N Param.: 12.70 sN Param.: 0.72 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
dimethylselenide (in CH2Cl2)  |
dichloromethane | N Param.: 12.60 sN Param.: 0.72 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
dibutylsulfide (in CH2Cl2)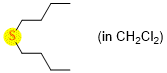  |
dichloromethane | N Param.: 11.86 sN Param.: 0.74 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
tetrahydrothiophene (in CH2Cl2)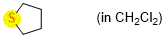  |
dichloromethane | N Param.: 13.10 sN Param.: 0.72 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
tetrahydrothiophene (in MeCN)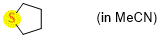  |
MeCN | N Param.: 13.30 sN Param.: 0.72 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
tetrahydrothiopyran (in CH2Cl2)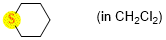  |
dichloromethane | N Param.: 11.94 sN Param.: 0.75 | Chem. Eur. J. 2021, 21, 11367-11376 10.1002/chem.202100977 | |
4-nitrothiophenolate (in DMSO)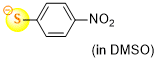  |
DMSO | N Param.: 18.92 sN Param.: 0.87 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
3,5-bis(trifluoromethyl)thiophenolate (in DMSO)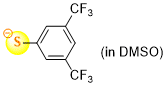  |
DMSO | N Param.: 19.71 sN Param.: 0.86 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
4-(trifluoromethyl)thiophenolate (in DMSO)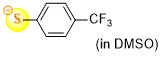  |
DMSO | N Param.: 21.30 sN Param.: 0.86 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
3-(trifluoromethyl)thiophenolate (in DMSO)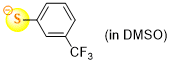  |
DMSO | N Param.: 21.75 sN Param.: 0.86 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
3-chlorothiophenolate (in DMSO)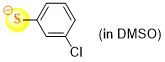  |
DMSO | N Param.: 22.50 sN Param.: 0.78 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
4-bromothiophenolate (in DMSO)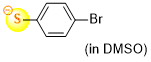  |
DMSO | N Param.: 22.80 sN Param.: 0.78 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
thiophenolate (in DMSO)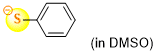  |
DMSO | N Param.: 23.36 sN Param.: 0.74 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
1,3-dimethyl-2-methyleneimidazolidine (in THF)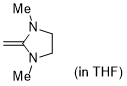  |
THF | N Param.: 18.11 sN Param.: 0.68 | J. Org. Chem. 2021, 86, 2974-2985 10.1021/acs.joc.0c02838 | |
1,3-dimethyl-2-methylenehexahydropyrimidine (in THF)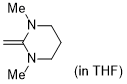  |
THF | N Param.: 18.68 sN Param.: 0.63 | J. Org. Chem. 2021, 86, 2974-2985 10.1021/acs.joc.0c02838 | |
1,3-dimesityl-2-methylene-2,3-dihydro-1H-imidazole (in THF)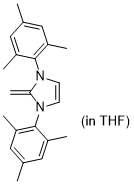  |
THF | N Param.: 17.80 sN Param.: 0.79 | J. Org. Chem. 2021, 86, 2974-2985 10.1021/acs.joc.0c02838 | |
1,3-dimethyl-2-methylene-2,3-dihydro-1H-benzo[d]imidazole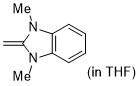  |
THF | N Param.: 19.84 sN Param.: 0.58 | J. Org. Chem. 2021, 86, 2974-2985 10.1021/acs.joc.0c02838 | |
4-methylthiophenolate (in DMSO)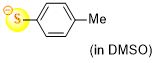  |
DMSO | N Param.: 24.35 sN Param.: 0.69 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
naphthalene-2-thiolate (in DMSO)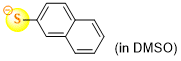  |
DMSO | N Param.: 22.55 sN Param.: 0.83 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
4-methoxythiophenolate (in DMSO)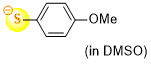  |
DMSO | N Param.: 24.97 sN Param.: 0.68 | J. Org. Chem. 2021, 86, 5965-5972 10.1021/acs.joc.1c00025 | |
prolinate (in MeCN)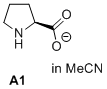  |
MeCN | N Param.: 19.95 sN Param.: 0.68 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 |
