Electrophiles
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
jul(t-Bu)2QM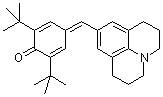  |
E Param.: -17.90 | Angew. Chem. Int. Ed. 2002, 41, 91-95 10.1002/1521-3773(20[...] | ||
jul-indan-1,3-dione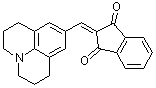  |
E Param.: -14.68 | Org. Biomol. Chem. 2007, 5, 3020-3026 10.1039/b708025e | ||
julBBS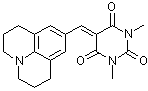  |
E Param.: -13.84 | J. Org. Chem. 2007, 72, 9170-9180 10.1021/jo071273g | ||
MacMillan-iminium ion (1st generation)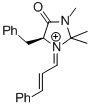  |
MeCN | E Param.: -7.37 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 10.1002/anie.201103683 | |
MacMillan-iminium ion (2nd generation)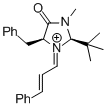  |
MeCN | E Param.: -5.52 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 10.1002/anie.201103683 | |
MacMillan-iminium ion (spiro)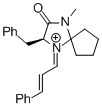  |
MeCN | E Param.: -7.67 | Angew. Chem. Int. Ed. 2011, 50, 9953-9956 10.1002/anie.201103683 | |
maleic anhydride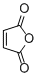  |
DMSO | E Param.: -11.31 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
Me2C=C=N(+)Me2  |
E Param.: -2.80 | Synthesis 2025, 57, 3251-3262 10.1055/a-2649-1999 | ||
Me2N+=CH-CH-Ph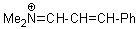  |
E Param.: -9.20 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
methoxy-(4-methoxyphenyl)methylium ion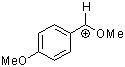  |
E Param.: 0.14 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
methoxy-(4-methylphenyl)methylium ion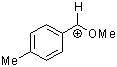  |
E Param.: 1.90 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
methoxy-phenylmethylium ion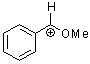  |
E Param.: 2.97 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
methyl diazoacetate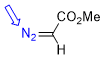  |
E Param.: -18.50 | Chem. Eur. J. 2022, 28, e202201376 10.1002/chem.202201376 | ||
methyl prop-2-enoate (in DMSO)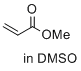  |
DMSO | E Param.: -18.84 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
methyl(phenyl)methyleneammonium ion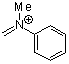  |
E Param.: -5.17 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
mor iminium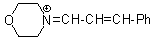  |
E Param.: -8.60 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
N,N-dimethylprop-2-enamide (in DMSO)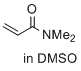  |
DMSO | E Param.: -23.54 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
N-(difluoromethyl)thio)phthalimide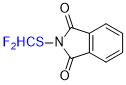  |
E Param.: -11.86 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
N-(phenylsulfonyl)-N-((trifluoromethyl)thio)benzenesulfonamide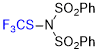  |
E Param.: -6.06 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
N-(trifluoromethyl)thio)phthalimide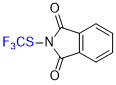  |
E Param.: -11.92 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
N-(trifluoromethyl)thio)saccharin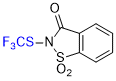  |
E Param.: -6.48 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
N-(trifluoromethyl)thio)succinimide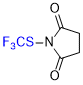  |
E Param.: -12.56 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
N-ethylidenecarbazolium ion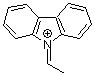  |
E Param.: 2.41 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
N-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate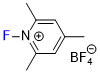  |
E Param.: -10.46 | J. Am. Chem. Soc. 2018, 140, 11474-11486 10.1021/jacs.8b07147 | ||
N-fluoro-2,6-dichloropyridinium tetrafluoroborate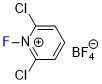  |
E Param.: -5.29 | J. Am. Chem. Soc. 2018, 140, 11474-11486 10.1021/jacs.8b07147 | ||
N-fluoropyridinium tetrafluoroborate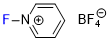  |
E Param.: -9.89 | J. Am. Chem. Soc. 2018, 140, 11474-11486 10.1021/jacs.8b07147 | ||
N-methyl maleimide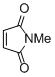  |
DMSO | E Param.: -14.07 | Eur. J. Org. Chem. 2014, , 2956-2963 10.1002/ejoc.201301779 | |
N-methyl-N-((trifluoromethyl)thio)-aniline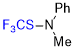  |
E Param.: -23.32 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
N-methyl-N-((trifluoromethyl)thio)-p-toluenesulfonamide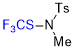  |
E Param.: -13.44 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
N-methylacridinium ion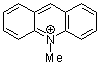  |
E Param.: -7.15 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
NFSI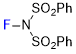  |
MeCN | E Param.: -8.44 | J. Am. Chem. Soc. 2018, 140, 11474-11486 10.1021/jacs.8b07147 | |
o-chloranil (C-Cl)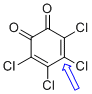  |
E Param.: -12.02 | J. Am. Chem. Soc. 2014, 136, 11499-11512 10.1021/ja505613b | ||
o-chloranil (C=O)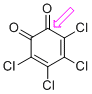  |
E Param.: -8.77 | J. Am. Chem. Soc. 2014, 136, 11499-11512 10.1021/ja505613b | ||
oxan-4-one (in DMSO)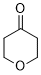  |
DMSO | E Param.: -17.90 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
p-(dimethylamino)benzylidene Meldrum's acid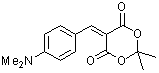  |
E Param.: -12.76 | J. Org. Chem. 2008, 73, 2738-2745 10.1021/jo702590s | ||
p-(dimethylamino)benzylidenemalononitrile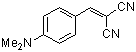  |
E Param.: -13.30 | J. Org. Chem. 2003, 68, 6880-6886 10.1021/jo0344182 | ||
p-(methoxy)benzylidene Meldrum's acid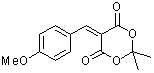  |
E Param.: -10.28 | J. Org. Chem. 2008, 73, 2738-2745 10.1021/jo702590s | ||
p-(methoxy)benzylidenemalononitrile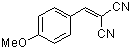  |
E Param.: -10.80 | J. Org. Chem. 2003, 68, 6880-6886 10.1021/jo0344182 | ||
p-AniCH=N+Me2 (in MeCN)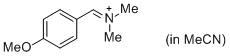  |
MeCN | E Param.: -10.69 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
p-CF3-C6H4-CH=N+Me2 (in MeCN)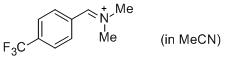  |
MeCN | E Param.: -8.34 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
p-chloranil (C-Cl)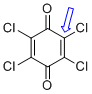  |
E Param.: -13.84 | J. Am. Chem. Soc. 2014, 136, 11499-11512 10.1021/ja505613b | ||
p-chloranil (C=O)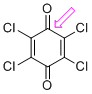  |
E Param.: -12.13 | J. Am. Chem. Soc. 2014, 136, 11499-11512 10.1021/ja505613b | ||
p-fluoranil (C-F)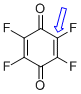  |
E Param.: -11.12 | J. Am. Chem. Soc. 2014, 136, 11499-11512 10.1021/ja505613b | ||
p-fluoranil (C=O)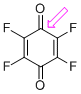  |
E Param.: -9.37 | J. Am. Chem. Soc. 2014, 136, 11499-11512 10.1021/ja505613b | ||
p-nitro-cinnamaldehyde (in DMSO)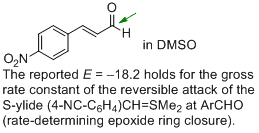  |
DMSO | J. Am. Chem. Soc. 2011, 133, 8240-8251 10.1021/ja200820m | ||
p-nitrophenyl isothiocyanate  |
DMSO | E Param.: -15.89 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
p-quinone (C-H)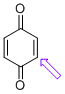  |
E Param.: -16.19 | J. Am. Chem. Soc. 2014, 136, 11499-11512 10.1021/ja505613b | ||
p-tosyl isocyanate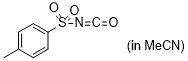  |
MeCN | E Param.: -7.69 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
Pd-allyl cation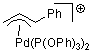  |
E Param.: -10.11 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
pentan-2-one (in DMSO)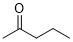  |
DMSO | E Param.: -22.30 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
pfp(Ph)CH+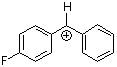  |
E Param.: 5.20 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | ||
Ph2C=C=N(+)Me2  |
E Param.: -1.90 | Synthesis 2025, 57, 3251-3262 10.1055/a-2649-1999 | ||
PhCH=N+(CH2)4 (in MeCN)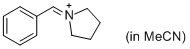  |
MeCN | E Param.: -9.35 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
PhCH=N+(CH2)5 (in MeCN)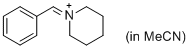  |
MeCN | E Param.: -9.60 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
PhCH=N+Me2 (in MeCN)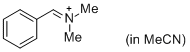  |
MeCN | E Param.: -9.27 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
phenyl isocyanate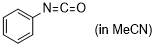  |
MeCN | E Param.: -15.38 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
phenyl isothiocyanate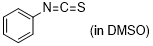  |
DMSO | E Param.: -18.15 | J. Am. Chem. Soc. 2020, 142, 8383-8402 10.1021/jacs.0c01960 | |
pipN+=CH-CH-Ph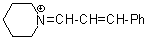  |
E Param.: -10.30 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
pop(Ph)CH+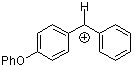  |
E Param.: 2.90 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | ||
pop(tol)CH+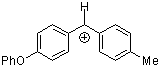  |
E Param.: 2.16 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
propyn-1-ylium-Co2(CO)6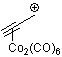  |
E Param.: -0.84 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
pTolCH=N+CH2 (in MeCN)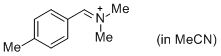  |
MeCN | E Param.: -9.64 | J. Am. Chem. Soc. 2013, 135, 6579-6587 10.1021/ja401106x | |
pyrN+=CH-CH-Ph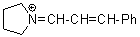  |
E Param.: -9.80 | Angew. Chem. Int. Ed. 2008, 47, 8723-8726 10.1002/anie.200802889 | ||
Selectfluor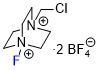  |
E Param.: -5.20 | J. Am. Chem. Soc. 2018, 140, 11474-11486 10.1021/jacs.8b07147 | ||
tert-butyl prop-2-enoate (in DMSO)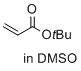  |
DMSO | E Param.: -20.22 | J. Am. Chem. Soc. 2017, 139, 13318-13329 10.1021/jacs.7b05106 | |
thian-4-one (in DMSO)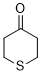  |
DMSO | E Param.: -16.90 | J. Am. Chem. Soc. 2018, 140, 5500-5515 10.1021/jacs.8b01657 | |
tol(Ph)CH+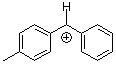  |
E Param.: 4.43 | J. Am. Chem. Soc. 2012, 134, 13902-13911 10.1021/ja306522b | ||
tol(t-Bu)2QM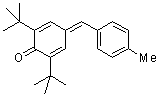  |
E Param.: -15.83 | Angew. Chem. Int. Ed. 2002, 41, 91-95 10.1002/1521-3773(20[...] | ||
tritylium ion (Ph3C+)  |
E Param.: 0.51 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | ||
tropylium ion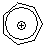  |
E Param.: -3.72 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
Umemoto I (tetrafluoroborate)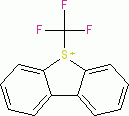  |
E Param.: -13.39 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
Umemoto I (triflate)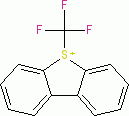  |
E Param.: -13.08 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
Umemoto II (triflate)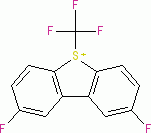  |
E Param.: -12.80 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
Vilsmeier ion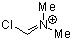  |
E Param.: -5.77 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | ||
[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = 3,5-difluorophenyl)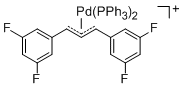  |
E Param.: -14.21 | Organometallics 2012, 31, 2416-2424 10.1021/om3000357 | ||
[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = 4-(dimethylamino)phenyl)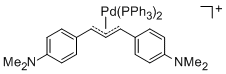  |
E Param.: -14.46 | Organometallics 2012, 31, 2416-2424 10.1021/om3000357 | ||
[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = Ph)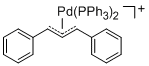  |
E Param.: -14.14 | Organometallics 2012, 31, 2416-2424 10.1021/om3000357 |
